Abstract
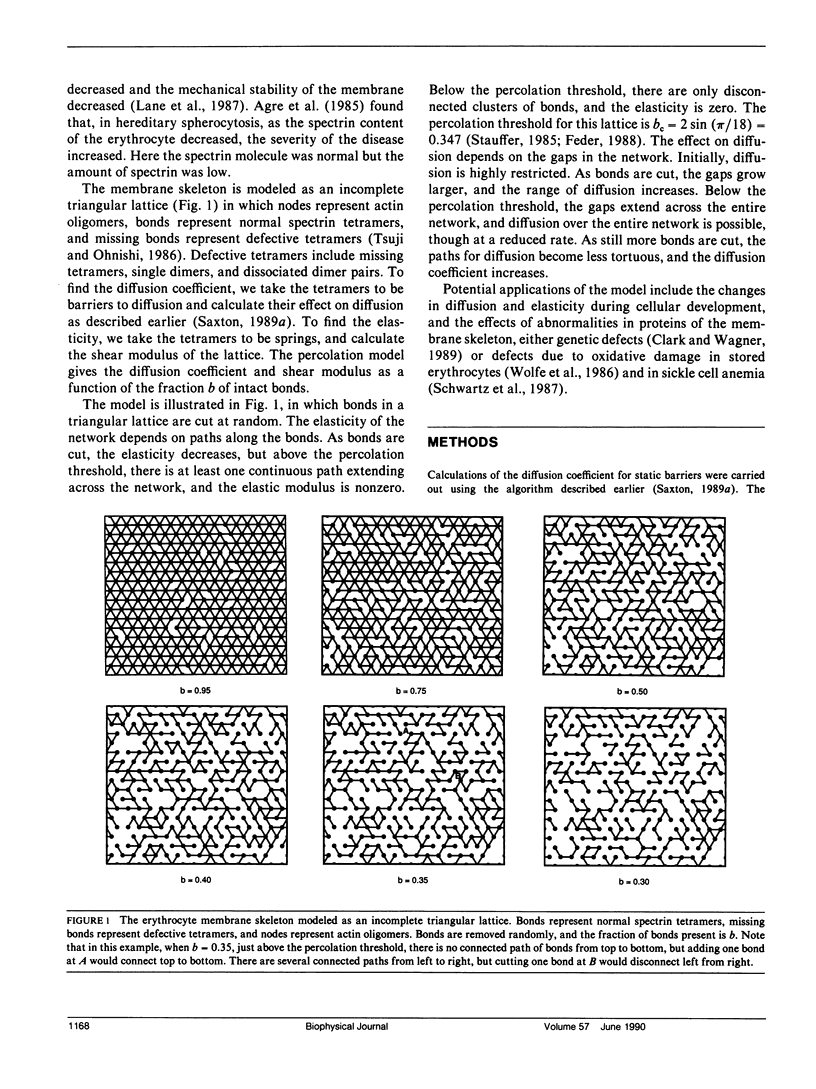
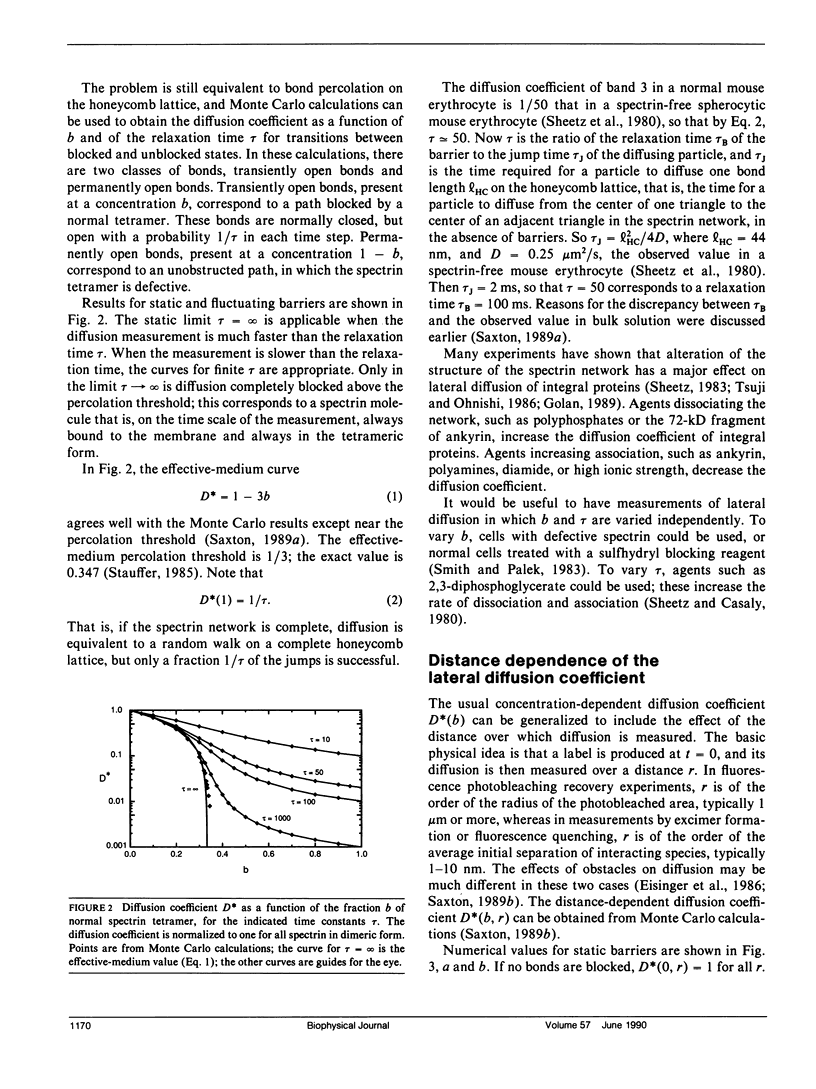
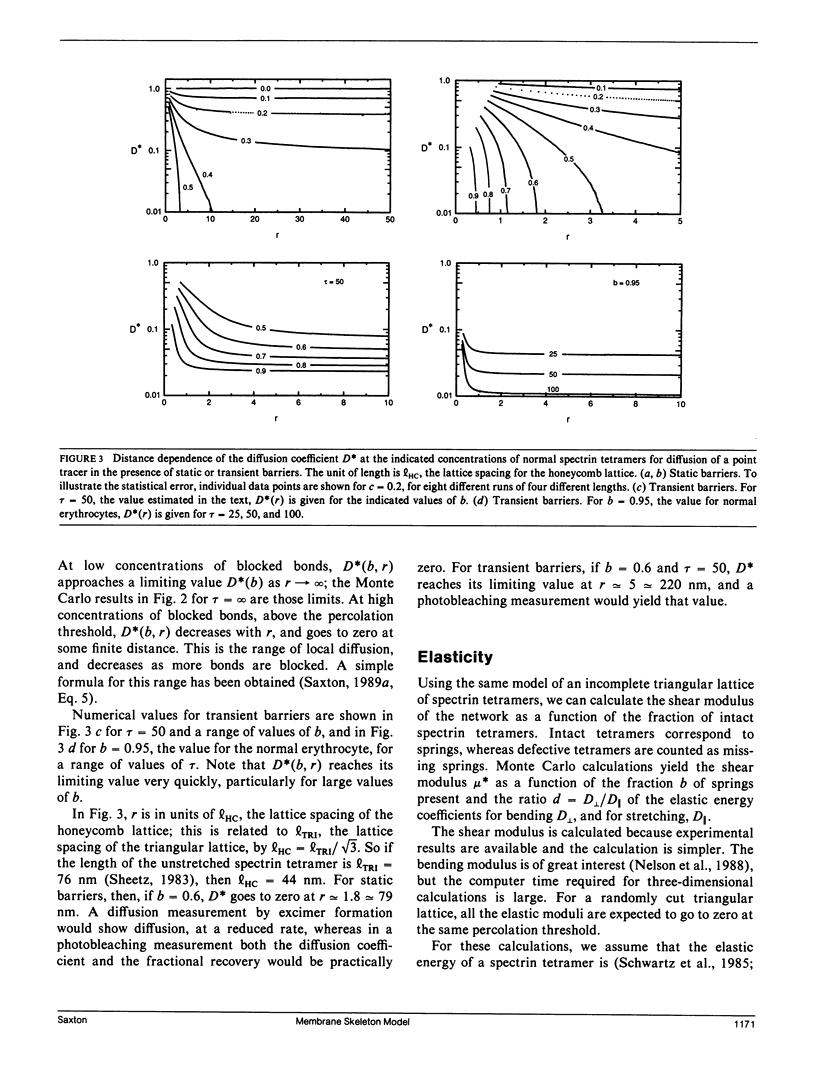
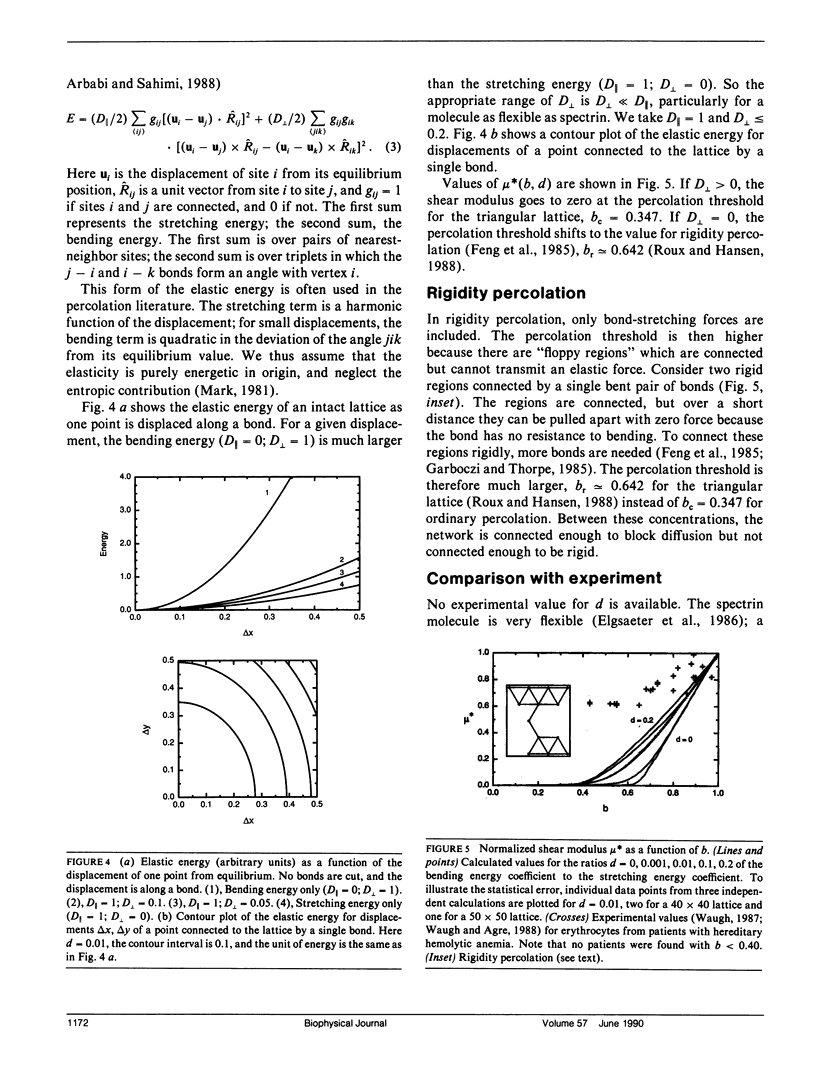
The spectrin network on the cytoplasmic surface of the erythrocyte membrane is modeled as a triangular lattice of spectrin tetramers. This network obstructs lateral diffusion of proteins and provides mechanical reinforcement to the membrane. These effects are treated in a systematic and unified manner in terms of a percolation model. The diffusion coefficient is obtained as a function of the fraction of normal spectrin tetramers for both static and fluctuating barriers. The elasticity of the network is calculated as a function of the fraction of normal spectrin and the ratio of bending to stretching energies. For static barriers, elasticity and lateral diffusion are incompatible: if a network is connected enough to be elastic, it is connected enough to block long-range lateral diffusion. The elasticity and the force required for mechanical breakdown go to zero at the percolation threshold; experimental evidence suggests the existence of a stability threshold at or near the percolation threshold. The model is qualitatively applicable to other cells with membrane skeletons, such as epithelial cells, in which localization of membrane proteins is essential to differentiation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Almers W., Stirling C. Distribution of transport proteins over animal cell membranes. J Membr Biol. 1984;77(3):169–186. doi: 10.1007/BF01870567. [DOI] [PubMed] [Google Scholar]
- Arbabi S, Sahimi M. Elastic properties of three-dimensional percolation networks with stretching and bond-bending forces. Phys Rev B Condens Matter. 1988 Oct 1;38(10):7173–7176. doi: 10.1103/physrevb.38.7173. [DOI] [PubMed] [Google Scholar]
- Beale PD, Srolovitz DJ. Elastic fracture in random materials. Phys Rev B Condens Matter. 1988 Apr 1;37(10):5500–5507. doi: 10.1103/physrevb.37.5500. [DOI] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Bernstein S. E. Inherited hemolytic disease in mice: a review and update. Lab Anim Sci. 1980 Apr;30(2 Pt 1):197–205. [PubMed] [Google Scholar]
- Bloom J. A., Webb W. W. Lipid diffusibility in the intact erythrocyte membrane. Biophys J. 1983 Jun;42(3):295–305. doi: 10.1016/S0006-3495(83)84397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabanel A., Sung K. L., Rapiejko J., Prchal J. T., Palek J., Liu S. C., Chien S. Viscoelastic properties of red cell membrane in hereditary elliptocytosis. Blood. 1989 Feb;73(2):592–595. [PubMed] [Google Scholar]
- Chasis J. A., Agre P., Mohandas N. Decreased membrane mechanical stability and in vivo loss of surface area reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Aug;82(2):617–623. doi: 10.1172/JCI113640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Fishkind D. J., Mooseker M. S., Morrow J. S. Contributions of the beta-subunit to spectrin structure and function. Cell Motil Cytoskeleton. 1989;12(4):248–263. doi: 10.1002/cm.970120406. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Schlüter K., Allen D. P., Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985 Dec 13;230(4731):1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgsaeter A., Stokke B. T., Mikkelsen A., Branton D. The molecular basis of erythrocyte shape. Science. 1986 Dec 5;234(4781):1217–1223. doi: 10.1126/science.3775380. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Baines A. J., Hann I. M., Al-Hakim I., Knowles S. M., Hoffbrand A. V. Defective spectrin dimer-dimer association in a family with transfusion dependent homozygous hereditary elliptocytosis. Br J Haematol. 1983 Jun;54(2):163–172. doi: 10.1111/j.1365-2141.1983.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Feng S, Thorpe MF, Garboczi E. Effective-medium theory of percolation on central-force elastic networks. Phys Rev B Condens Matter. 1985 Jan 1;31(1):276–280. doi: 10.1103/physrevb.31.276. [DOI] [PubMed] [Google Scholar]
- Fleming T. P. Trapped by a skeleton--the maintenance of epithelial membrane domains. Bioessays. 1987 Oct;7(4):179–181. doi: 10.1002/bies.950070410. [DOI] [PubMed] [Google Scholar]
- Garboczi EJ, Thorpe MF. Effective-medium theory of percolation on central-force elastic networks. II. Further results. Phys Rev B Condens Matter. 1985 Jun 1;31(11):7276–7281. doi: 10.1103/physrevb.31.7276. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Krebs K. E., Whitfield C. F., Riederer B. M., Zagon I. S. Spectrin and related molecules. CRC Crit Rev Biochem. 1988;23(2):171–234. doi: 10.3109/10409238809088319. [DOI] [PubMed] [Google Scholar]
- JOE M., TEASDALE J. M., MILLER J. R. A new mutation (sph) causing neonatal jaundice in the house mouse. Can J Genet Cytol. 1962 Jun;4:219–225. doi: 10.1139/g62-026. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Yguerabide J. The lateral mobility of the (Na+,K+)-dependent ATPase in Madin-Darby canine kidney cells. J Cell Biol. 1986 Apr;102(4):1256–1263. doi: 10.1083/jcb.102.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob R., Zimmermann M., Schoner W., Drenckhahn D. Colocalization and coprecipitation of ankyrin and Na+,K+-ATPase in kidney epithelial cells. Eur J Cell Biol. 1988 Feb;45(2):230–237. [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M. The Wellcome lecture, 1988. Muscular dystrophy: a time of hope. Proc R Soc Lond B Biol Sci. 1989 Jun 22;237(1286):1–9. doi: 10.1098/rspb.1989.0032. [DOI] [PubMed] [Google Scholar]
- Lane P. A., Shew R. L., Iarocci T. A., Mohandas N., Hays T., Mentzer W. C. Unique alpha-spectrin mutant in a kindred with common hereditary elliptocytosis. J Clin Invest. 1987 Mar;79(3):989–996. doi: 10.1172/JCI112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987 Mar;104(3):527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. L. Dystrophin. The gene and its product. Nature. 1989 Jun 22;339(6226):584–586. doi: 10.1038/339584a0. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis, spherocytosis and related disorders: consequences of a deficiency or a mutation of membrane skeletal proteins. Blood Rev. 1987 Sep;1(3):147–168. doi: 10.1016/0268-960x(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- Peterson L. C., Dampier C., Coetzer T., Lawler J., White J., Palek J. Clinical and laboratory study of two Caucasian families with hereditary pyropoikilocytosis and hereditary elliptocytosis. Am J Clin Pathol. 1987 Jul;88(1):58–65. doi: 10.1093/ajcp/88.1.58. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The spectrin network as a barrier to lateral diffusion in erythrocytes. A percolation analysis. Biophys J. 1989 Jan;55(1):21–28. doi: 10.1016/S0006-3495(89)82776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LM, Feng S, Thorpe MF, Sen PN. Behavior of depleted elastic networks: Comparison of effective-medium and numerical calculations. Phys Rev B Condens Matter. 1985 Oct 1;32(7):4607–4617. doi: 10.1103/physrevb.32.4607. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Rybicki A. C., Heath R. H., Lubin B. H. Protein 4.1 in sickle erythrocytes. Evidence for oxidative damage. J Biol Chem. 1987 Nov 15;262(32):15666–15672. [PubMed] [Google Scholar]
- Shahbakhti F., Gratzer W. B. Analysis of the self-association of human red cell spectrin. Biochemistry. 1986 Oct 7;25(20):5969–5975. doi: 10.1021/bi00368a020. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Casaly J. 2,3-Diphosphoglycerate and ATP dissociate erythrocyte membrane skeletons. J Biol Chem. 1980 Oct 25;255(20):9955–9960. [PubMed] [Google Scholar]
- Sheetz M. P. Membrane skeletal dynamics: role in modulation of red cell deformability, mobility of transmembrane proteins, and shape. Semin Hematol. 1983 Jul;20(3):175–188. [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Shields M., La Celle P., Waugh R. E., Scholz M., Peters R., Passow H. Effects of intracellular Ca2+ and proteolytic digestion of the membrane skeleton on the mechanical properties of the red blood cell membrane. Biochim Biophys Acta. 1987 Nov 27;905(1):181–194. doi: 10.1016/0005-2736(87)90022-8. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Palek J. Sulfhydryl reagents induce altered spectrin self-association, skeletal instability, and increased thermal sensitivity of red cells. Blood. 1983 Dec;62(6):1190–1196. [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. Human erythrocyte spectrin dimer intrinsic viscosity: temperature dependence and implications for the molecular basis of the erythrocyte membrane free energy. Biochim Biophys Acta. 1985 Jun 11;816(1):102–110. doi: 10.1016/0005-2736(85)90398-0. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Kawasaki K., Ohnishi S., Merkle H., Kusumi A. Regulation of band 3 mobilities in erythrocyte ghost membranes by protein association and cytoskeletal meshwork. Biochemistry. 1988 Sep 20;27(19):7447–7452. doi: 10.1021/bi00419a041. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986 Oct 7;25(20):6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Jan;81(1):133–141. doi: 10.1172/JCI113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J. 1987 Mar;51(3):363–369. doi: 10.1016/S0006-3495(87)83358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. C., Byrne A. M., Lux S. E. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J Clin Invest. 1986 Dec;78(6):1681–1686. doi: 10.1172/JCI112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zail S. Clinical disorders of the red cell membrane skeleton. Crit Rev Oncol Hematol. 1986;5(4):397–453. doi: 10.1016/s1040-8428(86)80004-x. [DOI] [PubMed] [Google Scholar]


