Abstract
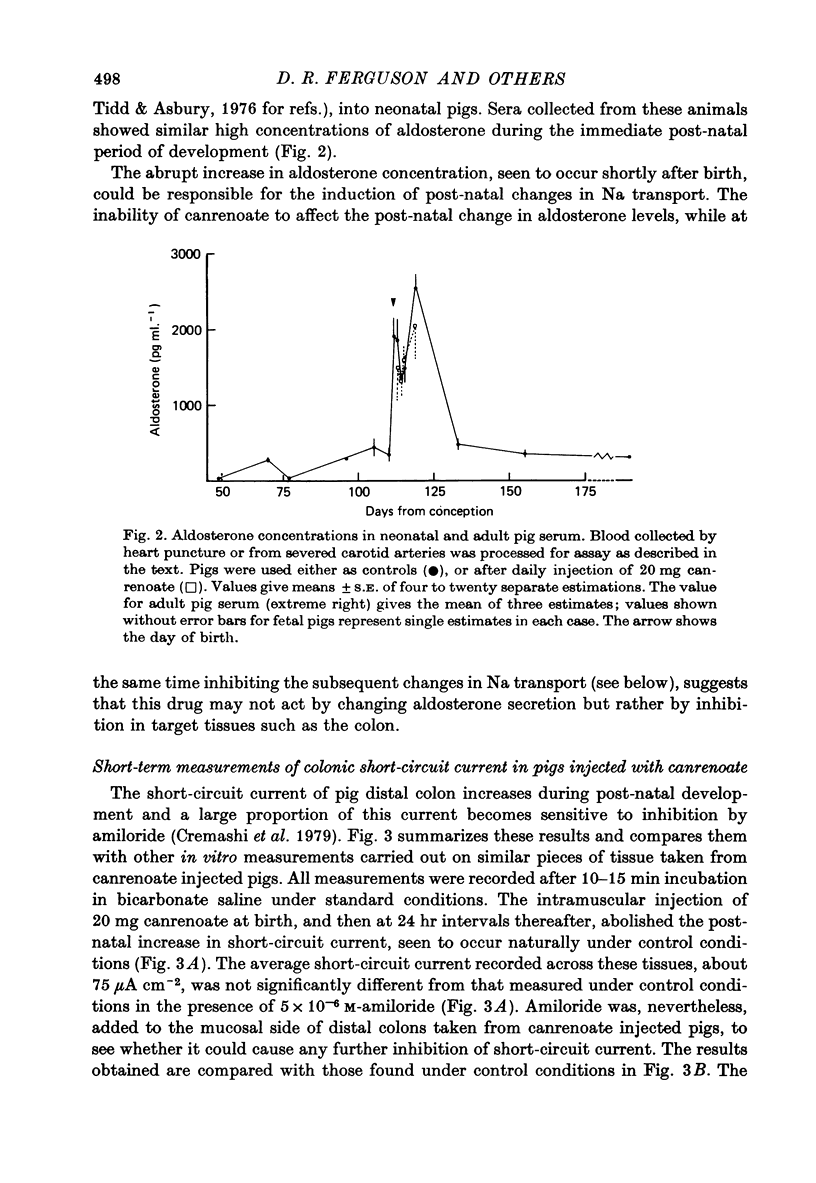
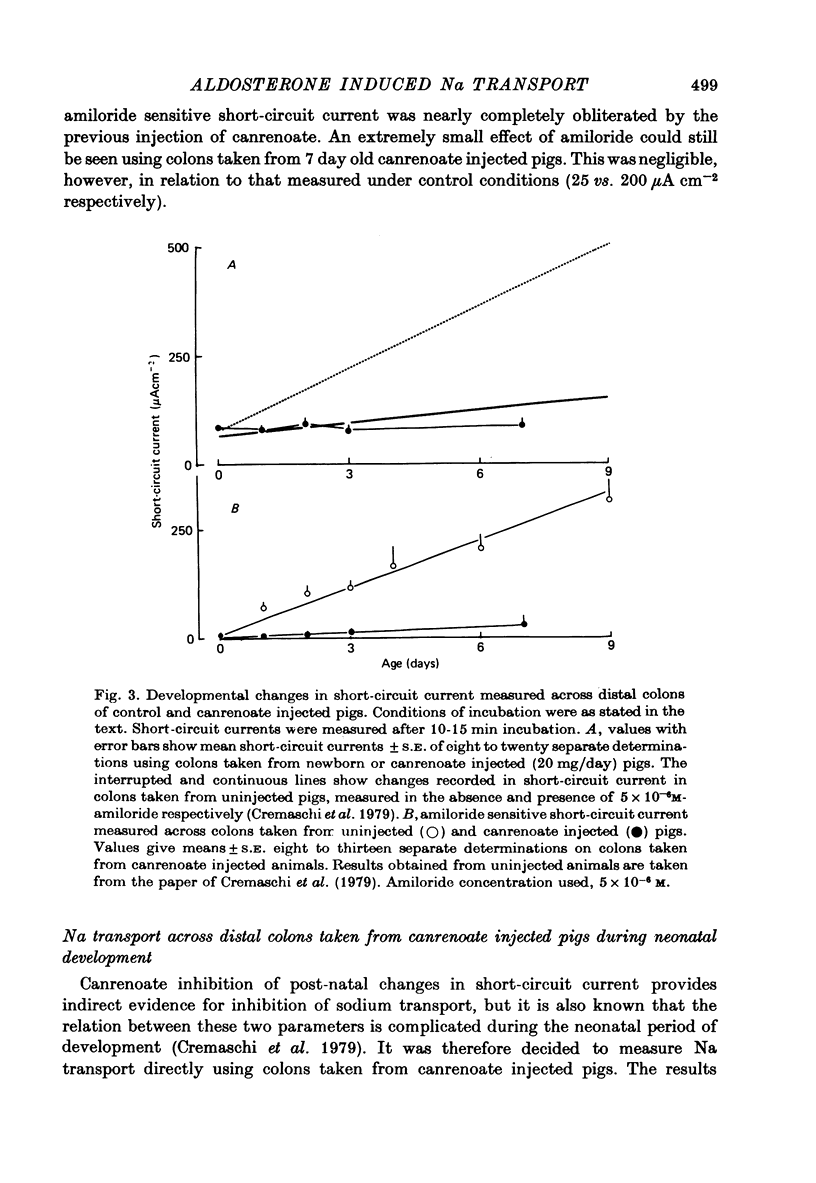
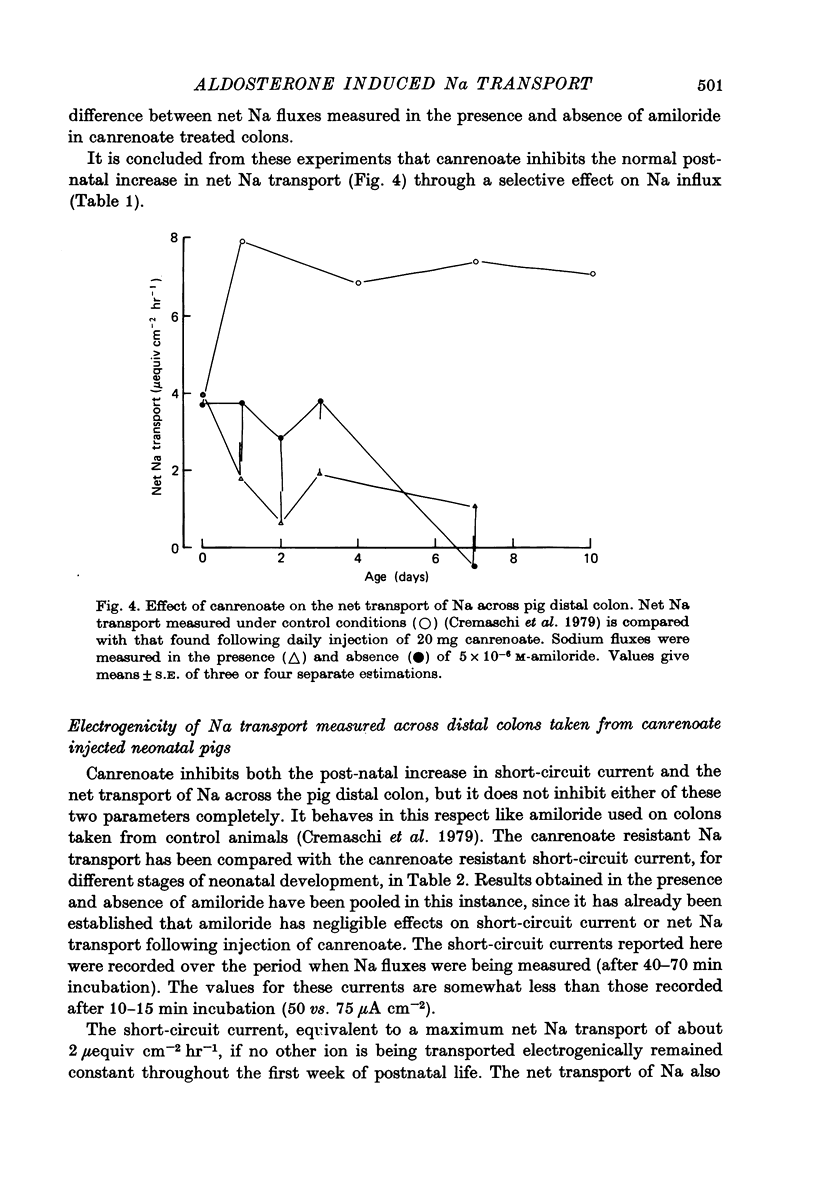
1. Serum concentrations of aldosterone in later fetal, 3-6 week old and adult pigs are of the order of 300 pg ml.-1. This increases to about 2000 pg ml.-1 in the period immediately after birth. 2. Canrenoate injected into pigs from birth onwards stops the increase in colonic short-circuit current, seen to take place normally during early postnatal development. Amiloride has little or no effect on the short-circuit current of colons taken from canrenoate injected pigs. 3. Canrenoate stops the post-natal increase in colonic Na influx (and therefore net transport) seen to occur under normal conditions. 4. There is in the neonatal pig distal colon a portion of Na transport which appears to be resistant to inhibition by amiloride or canrenoate. 5. There is a second portion of Na transport, increasing in importance as the piglets become older, which is electrogenic and which is electrogenic and which is inhibited by prior injection of canrenoate. It is assumed that this fraction of Na transport is influenced by aldosterone. 6. There is a third part of Na transport, maximal in colons taken from one day old animals, which appears to be non-electrogenic. This is also blocked by prior injection of canrenoate. 7. The physiological relevance of these findings is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P. J., Smith M. W. Transport of electrolytes across the helicoidal colon of the new-born pig. J Physiol. 1975 Jul;249(1):103–117. doi: 10.1113/jphysiol.1975.sp011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremaschi D., Ferguson D. R., Hénin S., James P. S., Meyer G., Smith M. W. Post-natal development of amiloride sensitive sodium transport in pig distal colon. J Physiol. 1979 Jul;292:481–494. doi: 10.1113/jphysiol.1979.sp012866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Okpako D., Shwm W. K. Proceedings: Aldosterone, moulting and the number of sodium channels in frog skin. Br J Pharmacol. 1974 May;51(1):128P–129P. [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Shum W. K. Estimation of the lifespan of amiloride binding sites in the membranes of toad bladder epithelial cells. J Physiol. 1976 Mar;255(3):605–618. doi: 10.1113/jphysiol.1976.sp011298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle M., Giry J., Gay M., Delost P. Perinatal changes in plasma and adrenal corticosterone and aldosterone concentrations in the mouse. J Endocrinol. 1978 Feb;76(2):303–309. doi: 10.1677/joe.0.0760303. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., BOREK F., BEISER S. M., LIEBERMAN S. Steroid-protein conjugates. I. Preparation and characterization of conjugates of bovine serum albumin with testosterone and with cortisone. J Biol Chem. 1957 Oct;228(2):713–727. [PubMed] [Google Scholar]
- Edmonds C. J., Godfrey R. C. Measurement of electrical potentials of the human rectum and pelvic colon in normal and aldosterone-treated patients. Gut. 1970 Apr;11(4):330–337. doi: 10.1136/gut.11.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. C. The effect of aldosterone and adrenalectomy on the electrical potential difference of rat colon and on the transport of sodium, potassium, chloride and bicarbonate. J Endocrinol. 1967 Dec;39(4):517–531. doi: 10.1677/joe.0.0390517. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Schultz S. G. Effect of aldosterone on ion transport by rabbit colon in vitro. J Membr Biol. 1978 Feb 6;39(1):1–26. doi: 10.1007/BF01872752. [DOI] [PubMed] [Google Scholar]
- Henriques de Jesus C., Smith M. W. Sodium transport by the small intestine of new-born and suckling pigs. J Physiol. 1974 Nov;243(1):211–224. doi: 10.1113/jphysiol.1974.sp010750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P. S., Smith M. W. Effect of ileostomy on changing transport function in the new-born pig colon [proceedings]. J Physiol. 1977 Oct;272(1):61P–62P. [PubMed] [Google Scholar]
- LEVITAN R., INGELFINGER F. J. EFFECT OF D-ALDOSTERONE ON SALT AND WATER ABSORPTION FROM THE INTACT HUMAN COLON. J Clin Invest. 1965 May;44:801–808. doi: 10.1172/JCI105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance R. A. The effect of age on the weights and lengths of pigs' intestines. J Anat. 1974 Jul;117(Pt 3):475–479. [PMC free article] [PubMed] [Google Scholar]
- Ramsay L., Shelton J., Harrison I., Tidd M., Asbury M. Spironolactone and potassium canrenoate in normal man. Clin Pharmacol Ther. 1976 Aug;20(2):167–177. doi: 10.1002/cpt1976202167. [DOI] [PubMed] [Google Scholar]
- Shields R., Mulholland A. T., Elmslie R. G. Action of aldosterone upon the intestinal transport of potassium, sodium, and water. Gut. 1966 Dec;7(6):686–696. doi: 10.1136/gut.7.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Permeability of the rabbit colon in vitro. Am J Physiol. 1977 Jan;232(1):F5–F9. doi: 10.1152/ajprenal.1977.232.1.F5. [DOI] [PubMed] [Google Scholar]


