Abstract
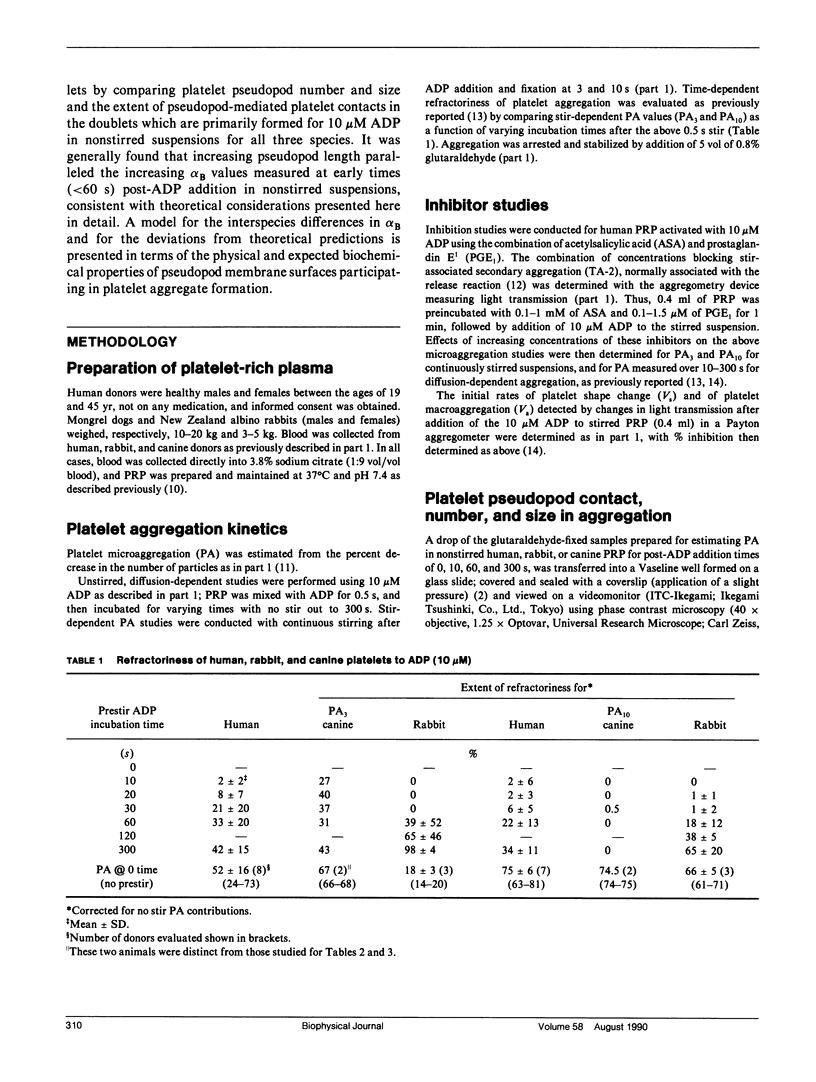
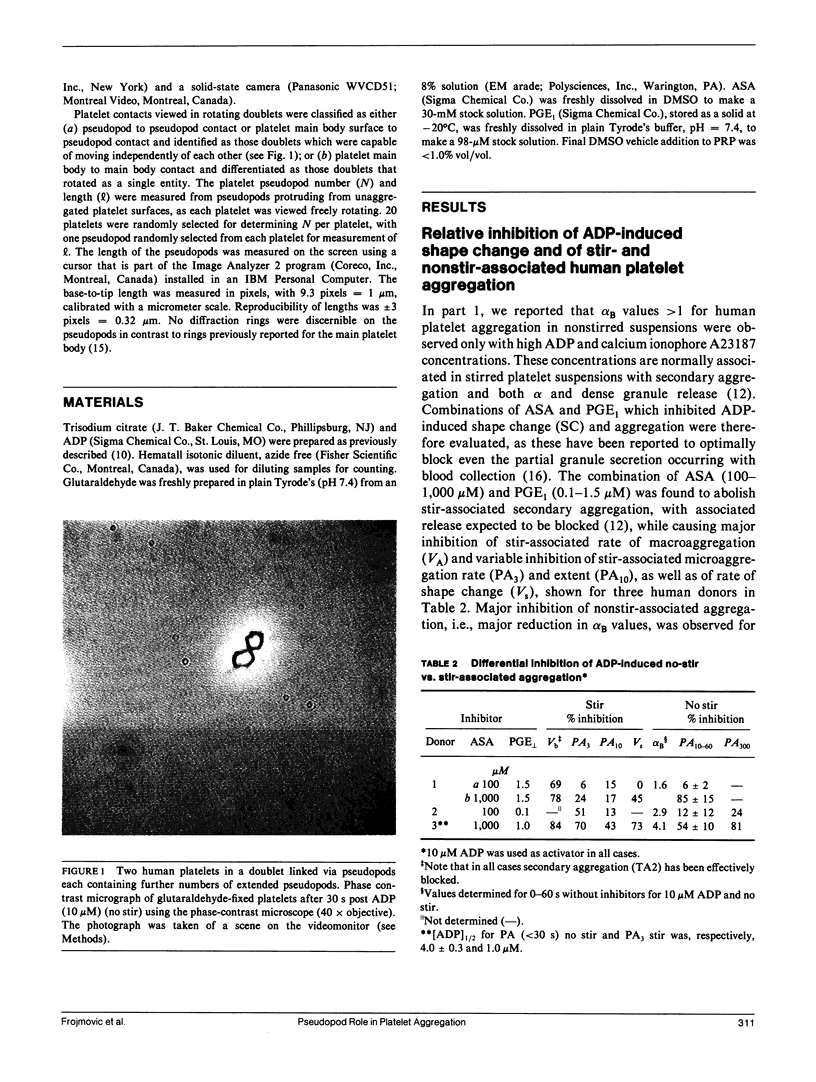
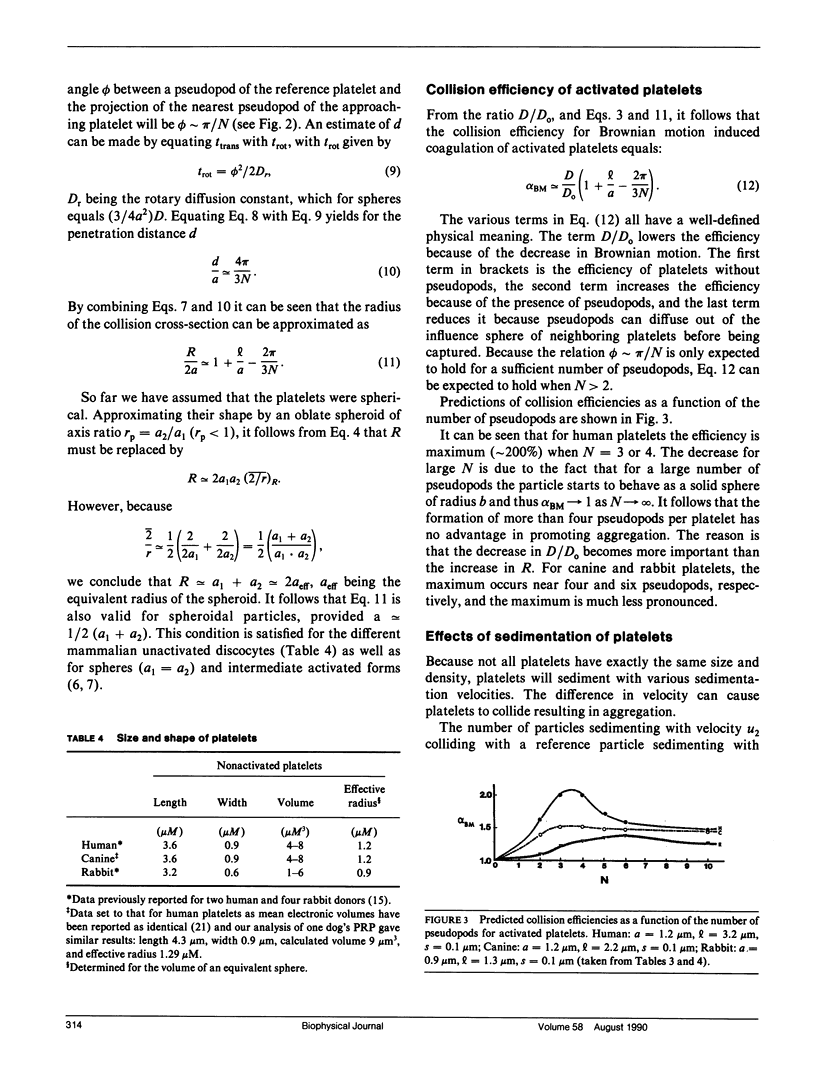
In part 1, we reported that human (H) platelets, activated with high concentrations (10 microM) of adenosine diphosphate, aggregate under Brownian diffusion (nonstirred, platelet-rich plasma) with an apparent efficiency of collision (alpha B) approximately 4 times and 8 times larger than observed, respectively, for canine (C) and rabbit (R) platelets. Further evaluations of parallel inhibition of alpha B and shape change suggested a central role for platelet pseudopods in mediating the long-range interactions associated with the elevated alpha B values. We found that greater than 90% of all platelet contacts in the doublets and triplets formed were via at least one pseudopod. We therefore compared pseudopod number and length per platelet generated by approximately 30 s post ADP activation in nonstirred PRP from human, canine, and rabbit donors, using phase-contrast, video-enhanced microscopy of fixed platelets. Theoretical calculations assessing the effects of pseudopod length and number on the collision frequency enhanced by an increased radius of a collision sphere supported the experimental observations that approximately 3 or 4 pseudopods per human or canine platelet, and approximately 5 or 6 pseudopods per rabbit platelet yield optimal alpha B values, with the average pseudopod length: approximately 3:2:1 for H/C/R, paralleling the alpha B differences. After correcting for effects of pseudopods and platelet size on platelet diffusion and sedimentation, it still appeared that the small number of long pseudopods formed on human platelets could largely explain the unusually large alpha B values. The quantitative discrepancies between theory and experiment do not appear related to time-dependent refractoriness within the less than 60 s of observation, but may be related to biochemical differences in dynamics and surface density of adhesive (sticky sites) present on the pseudopod surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Zacharski L. R., Widirstky S. T., Rosenstein R., Zaitlin L. M., Burgess D. R. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol. 1979 Oct;83(1):126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files J. C., Malpass T. W., Yee E. K., Ritchie J. L., Harker L. A. Studies of human plate alpha-granule release in vivo. Blood. 1981 Sep;58(3):607–618. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Duchastel A. Microscopic measurements of platelet aggregation reveal a low ADP-dependent process distinct from turbidometrically measured aggregation. J Lab Clin Med. 1983 Jun;101(6):964–976. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Gear A. L. Platelet aggregation measured in vitro by microscopic and electronic particle counting. Methods Enzymol. 1989;169:134–149. doi: 10.1016/0076-6879(89)69055-6. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G. Human platelet size, shape, and related functions in health and disease. Physiol Rev. 1982 Jan;62(1):185–261. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G. Physical, chemical and functional changes following platelet activation in normal and "giant" platelets. Blood Cells. 1983;9(2):359–382. [PubMed] [Google Scholar]
- Frojmovic M. M., Panjwani R. Geometry of normal mammalian platelets by quantitative microscopic studies. Biophys J. 1976 Sep;16(9):1071–1089. doi: 10.1016/S0006-3495(76)85756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., Albrecht R. M. Redistribution of the fibrinogen receptor of human platelets after surface activation. J Cell Biol. 1984 Sep;99(3):822–829. doi: 10.1083/jcb.99.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. A., Jones G. E. Microspike function in cell aggregation. Eur J Cell Biol. 1982 Oct;28(2):278–280. [PubMed] [Google Scholar]
- Milton J. G., Frojmovic M. M. Adrenaline and adenosine diphosphate-induced platelet aggregation require shape change. Importance of pseudopods. J Lab Clin Med. 1984 Nov;104(5):805–815. [PubMed] [Google Scholar]
- Milton J. G., Frojmovic M. M. Unusual properties of platelet shape in coronary and cerebral artery disease. Thromb Res. 1987 Sep 1;47(5):511–531. doi: 10.1016/0049-3848(87)90357-4. [DOI] [PubMed] [Google Scholar]
- Nakeff A., Ingram M. Platelet count: volume relationships in four mammalian species. J Appl Physiol. 1970 Apr;28(4):530–533. doi: 10.1152/jappl.1970.28.4.530. [DOI] [PubMed] [Google Scholar]
- Oliver J. A., Albrecht R. M. Colloidal gold labelling of fibrinogen receptors in epinephrine- and ADP-activated platelet suspensions. Scanning Microsc. 1987 Jun;1(2):745–756. [PubMed] [Google Scholar]
- Pedvis L. G., Wong T., Frojmovic M. M. Differential inhibition of the platelet activation sequence: shape change, micro- and macro-aggregation, by a stable prostacyclin analogue (Iloprost). Thromb Haemost. 1988 Apr 8;59(2):323–328. [PubMed] [Google Scholar]
- Tang S. S., Frojmovic M. M. The effects of pCO2 and pH on platelet shape change and aggregation for human and rabbit platelet-rich plasma. Thromb Res. 1977 Jan;10(1):135–145. doi: 10.1016/0049-3848(77)90086-x. [DOI] [PubMed] [Google Scholar]
- Weiss L. Cellular locomotive pressure in relation to initial cell contacts. J Theor Biol. 1964 Mar;6(2):275–281. doi: 10.1016/0022-5193(64)90033-5. [DOI] [PubMed] [Google Scholar]



