Abstract
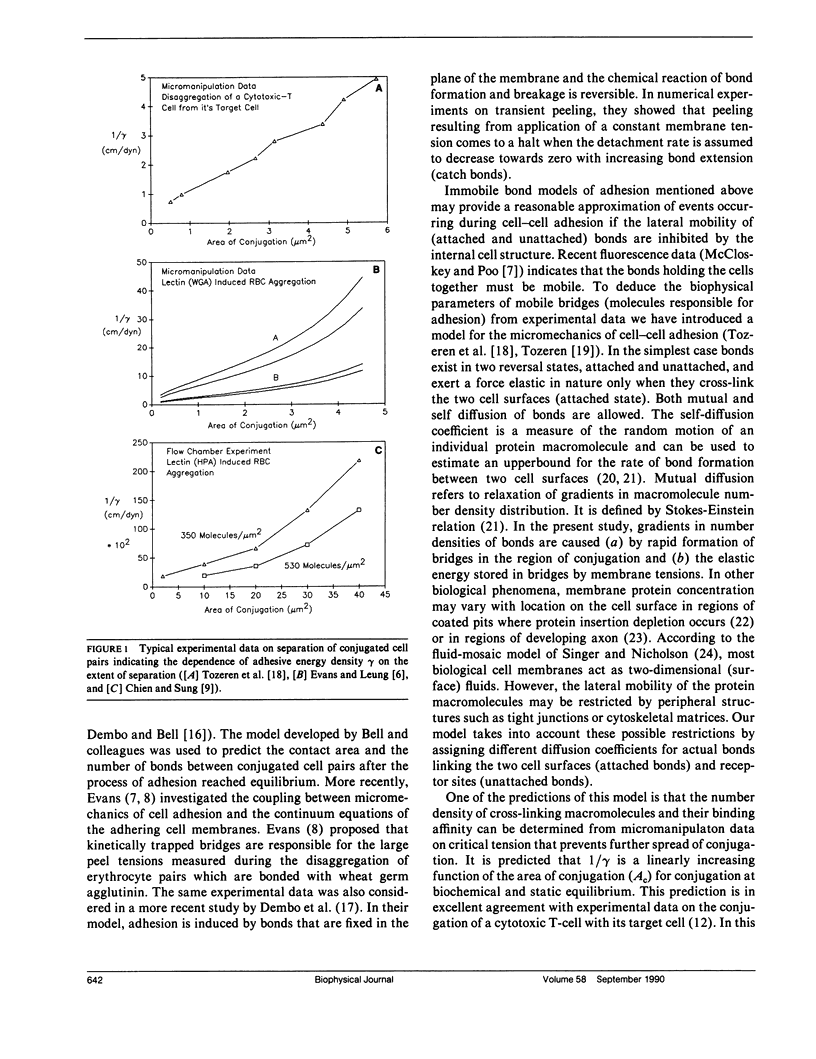
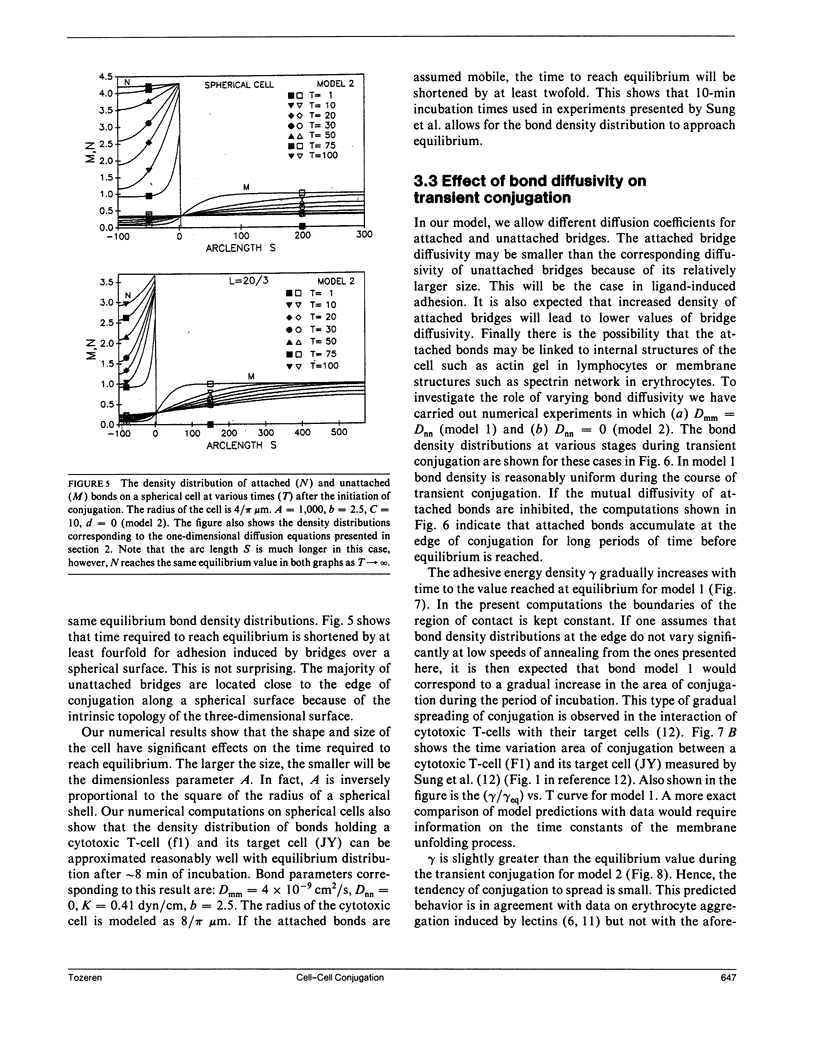
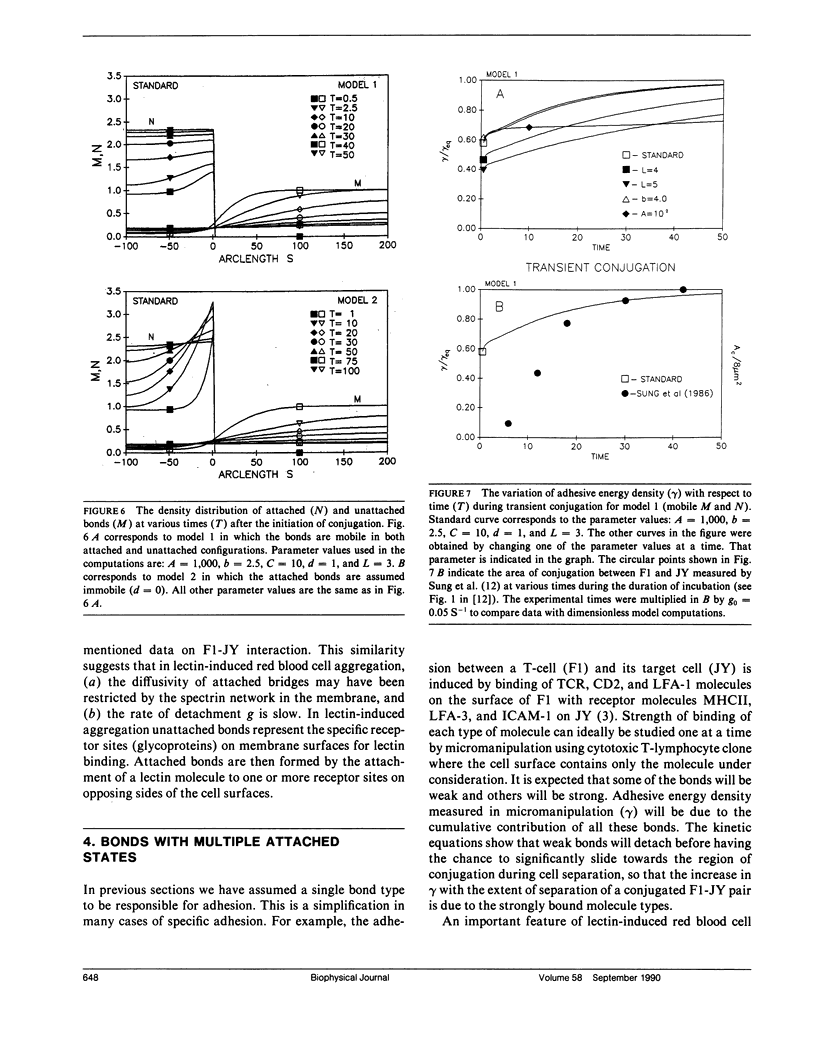
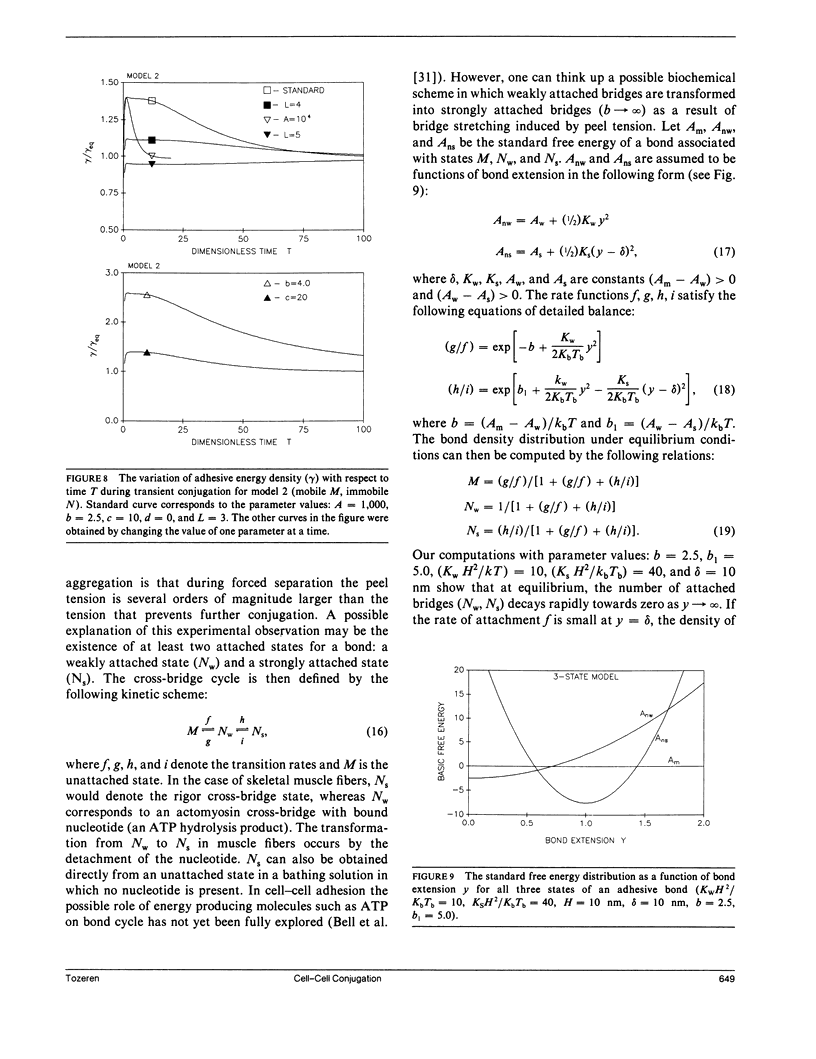
In the present study we investigate the transient conjugation of cell pairs by using a mathematical model. Macromolecules responsible for adhesion (bonds) are assumed to exist in two reversible states, attached and unattached, and exert a force elastic in nature only when they cross-link the two cell surfaces (attached state). Bonds form a link between the two cell surfaces only in the attached form. The unattached bridges are assumed laterally mobile in the plane of the cell membrane. Lateral mobility of attached bonds may be limited by structures on the undersurface of the cell membrane. Using this model we show that the bond density distribution between a cytotoxic T-cell (F-1) and a cancer cell (JY:HLA-A2-B7-DR4, W6) approaches equilibrium within 10 min, the incubation period used in experiments by Sung, K.L.P., L.A. Sung, M. Crimmins, S.J. Burakoff, and S. Chien (1986. Science [Wash. DC]. 234:1405-1408). If the diffusion coefficient of attached bonds is set equal to zero in the computations the model predictions indicate accumulation of bonds at the edge of conjugation. This prediction is consistent with present experimental data on lectin-induced red blood cell aggregation (Vayo, M., R. Skalak, P. Brunn, S. Usami, and S. Chien. 1987. Fed. Proc. 46:1043). It is concluded that significant features of micromanipulation data on specific adhesion can be explained by the diffusivity properties of bonds responsible for adhesion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Mutual diffusion of interacting membrane proteins. Biophys J. 1989 Aug;56(2):315–326. doi: 10.1016/S0006-3495(89)82678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Halperin B. I. Effects of spatial variation in membrane diffusibility and solubility on the lateral transport of membrane components. Biophys J. 1986 Sep;50(3):513–521. doi: 10.1016/S0006-3495(86)83489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys J. 1985 Jul;48(1):175–183. doi: 10.1016/S0006-3495(85)83770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. II. Discrete kinetically trapped molecular cross-bridges. Biophys J. 1985 Jul;48(1):185–192. doi: 10.1016/S0006-3495(85)83771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Buxbaum K. Affinity of red blood cell membrane for particle surfaces measured by the extent of particle encapsulation. Biophys J. 1981 Apr;34(1):1–12. doi: 10.1016/S0006-3495(81)84834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Ettensohn C. A. Cell adhesion in morphogenesis. Annu Rev Cell Biol. 1987;3:319–345. doi: 10.1146/annurev.cb.03.110187.001535. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Poo M. M. Contact-induced redistribution of specific membrane components: local accumulation and development of adhesion. J Cell Biol. 1986 Jun;102(6):2185–2196. doi: 10.1083/jcb.102.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Small R. K., Blank M., Ghez R., Pfenninger K. H. Components of the plasma membrane of growing axons. II. Diffusion of membrane protein complexes. J Cell Biol. 1984 Apr;98(4):1434–1443. doi: 10.1083/jcb.98.4.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986 Dec 12;234(4782):1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- Tozeren A. Adhesion induced by mobile cross-bridges: steady state peeling of conjugated cell pairs. J Theor Biol. 1989 Sep 11;140(1):1–17. doi: 10.1016/s0022-5193(89)80025-6. [DOI] [PubMed] [Google Scholar]
- Tozeren A., Sung K. L., Chien S. Theoretical and experimental studies on cross-bridge migration during cell disaggregation. Biophys J. 1989 Mar;55(3):479–487. doi: 10.1016/S0006-3495(89)82841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozeren A. The influence of doubly attached crossbridges on the mechanical behavior of skeletal muscle fibers under equilibrium conditions. Biophys J. 1987 Nov;52(5):901–906. doi: 10.1016/S0006-3495(87)83284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözeren A., Schoenberg M. The effect of cross-bridge clustering and head-head competition on the mechanical response of skeletal muscle under equilibrium conditions. Biophys J. 1986 Nov;50(5):875–884. doi: 10.1016/S0006-3495(86)83528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


