Abstract
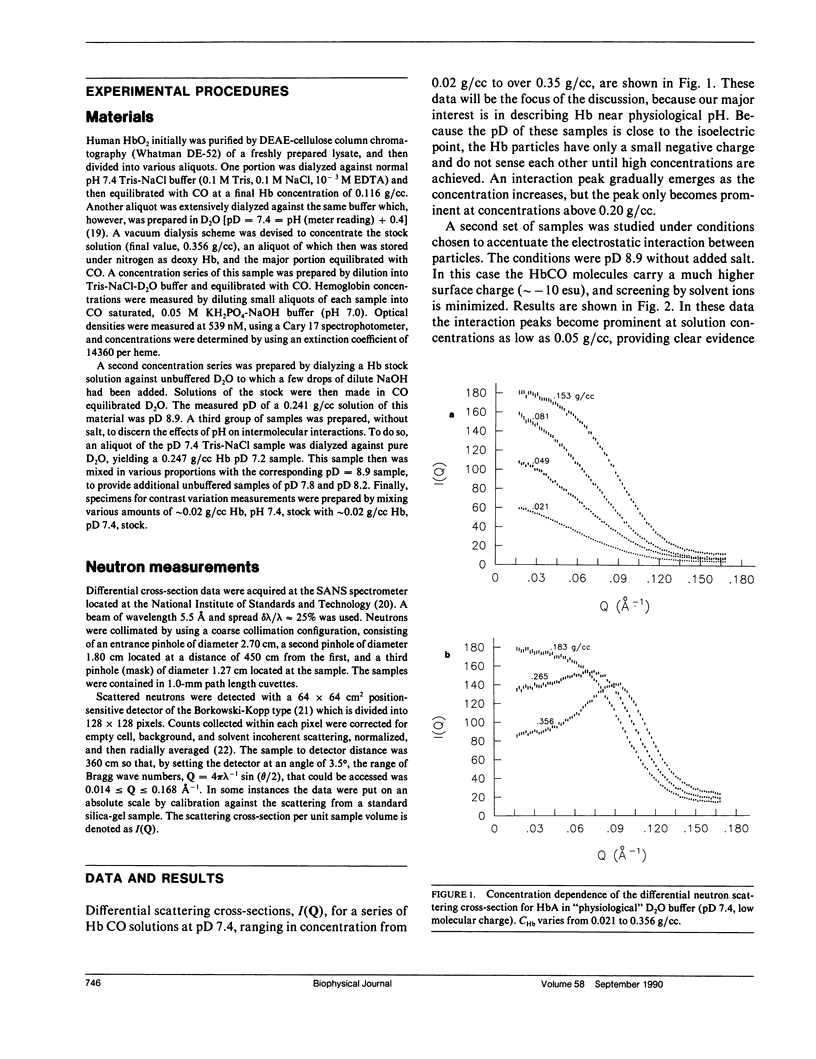
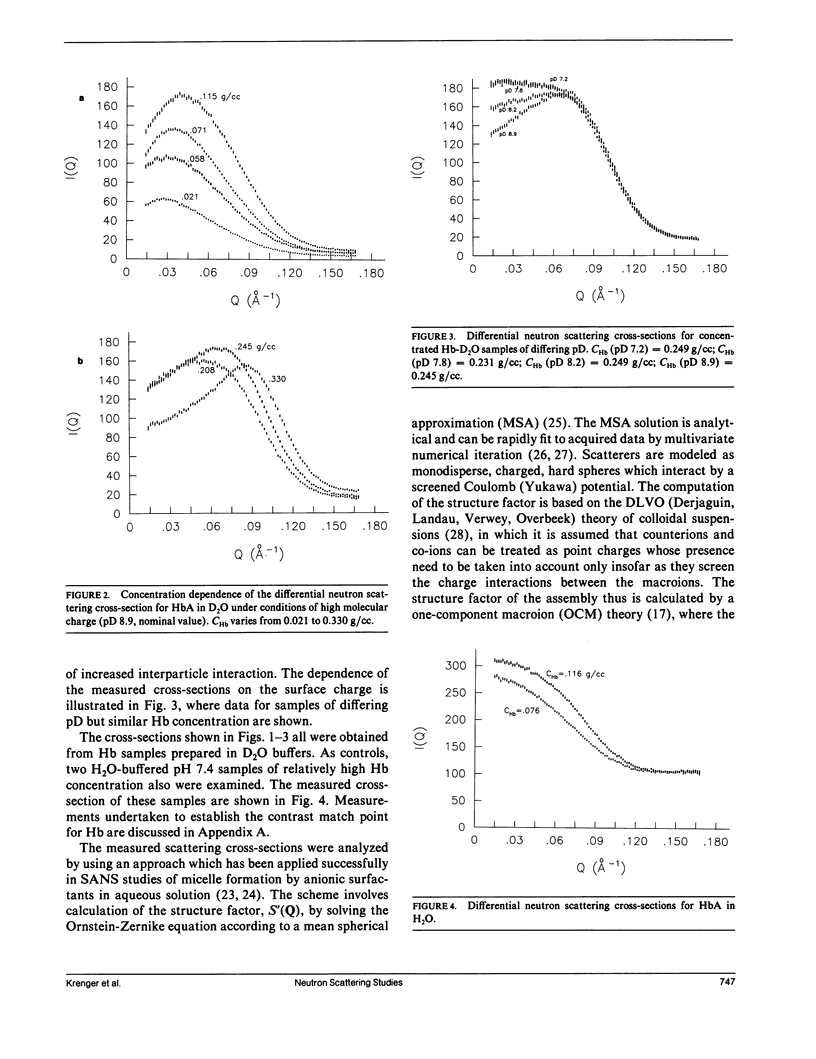
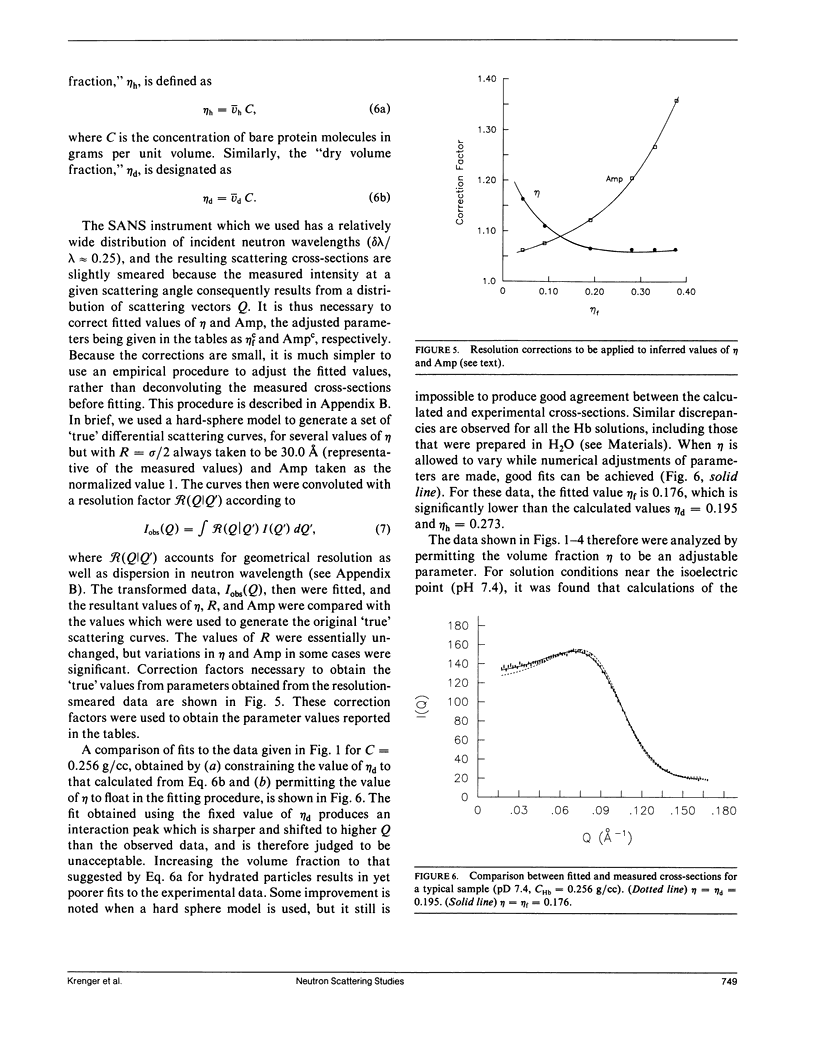
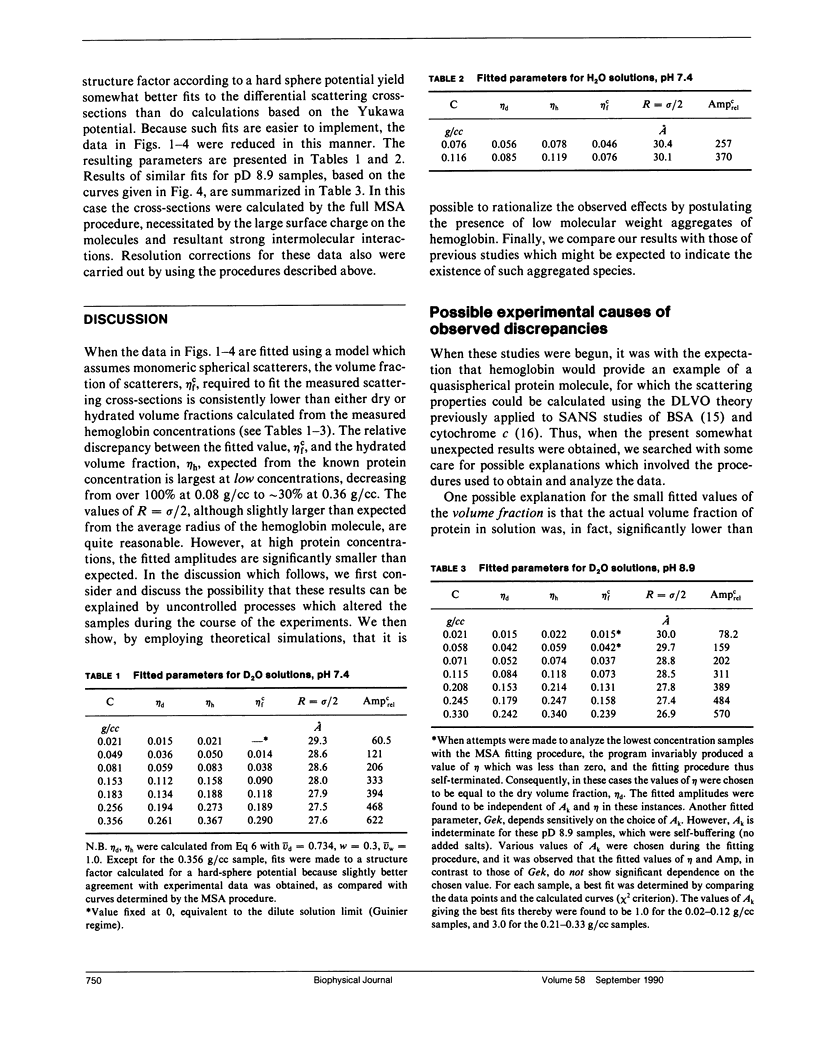
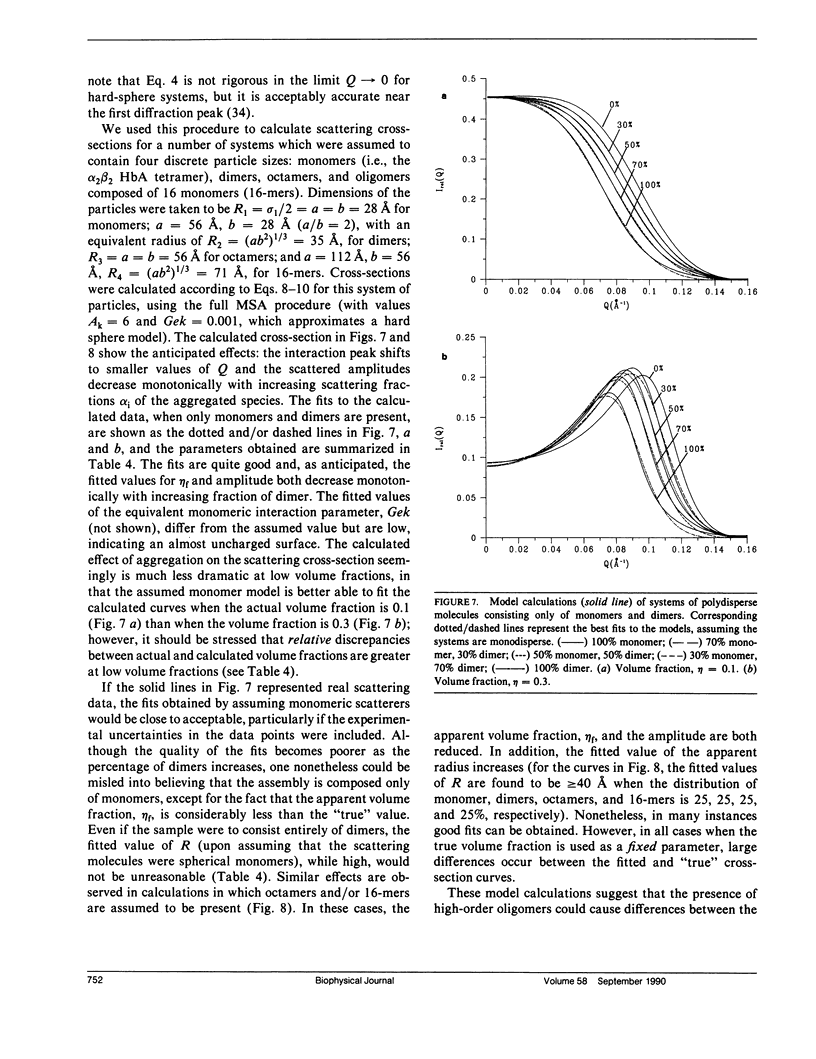
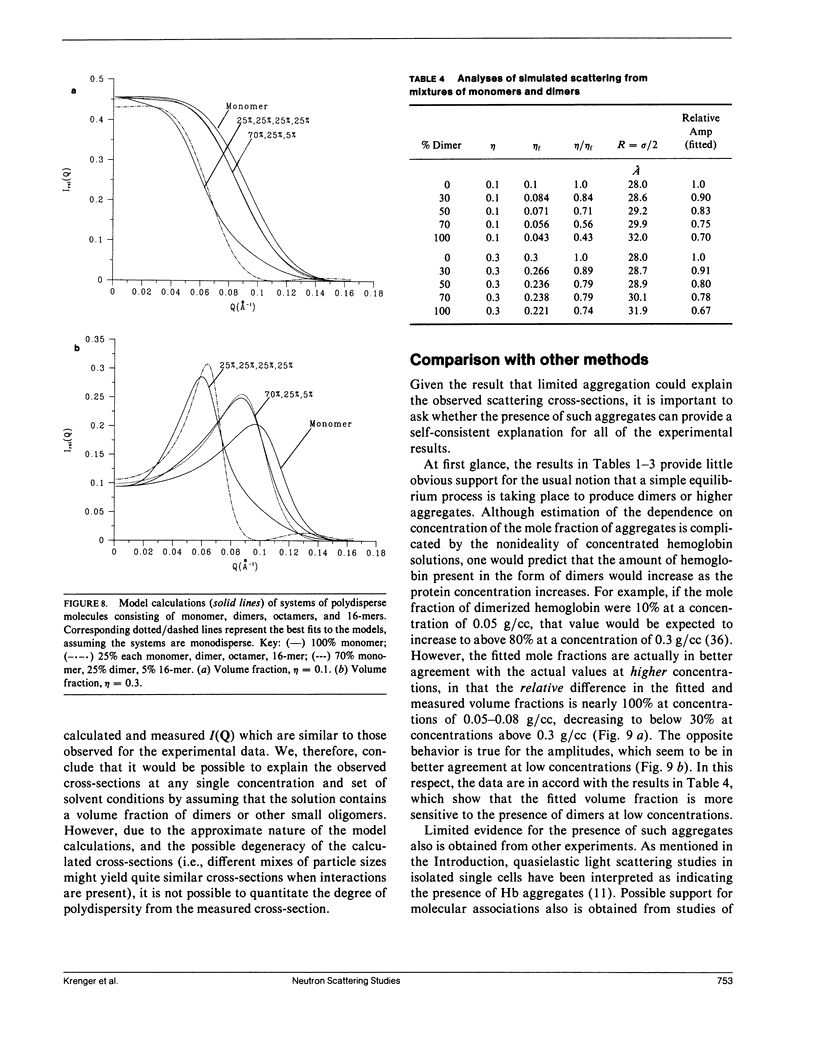
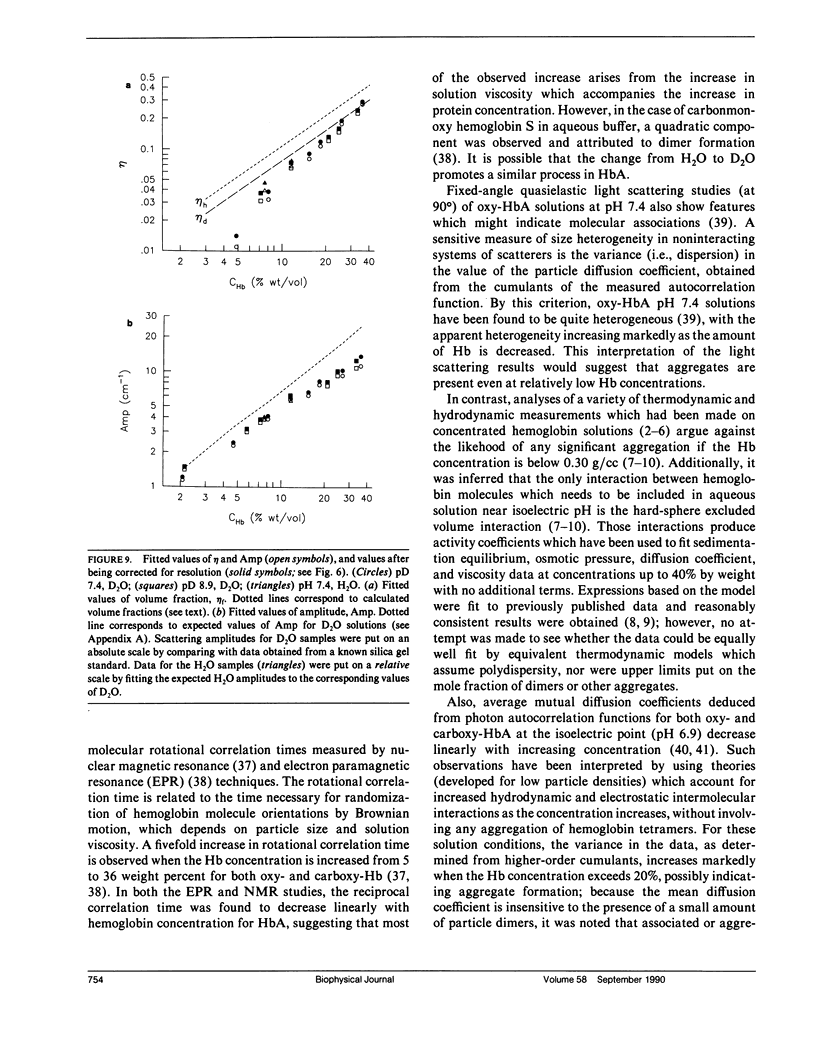
Differential cross-sections for neutrons scattered by normal human hemoglobin have been determined over the range of concentrations from 2 to approximately 35 weight percent. Data are compared with structure factors calculated from models of monodisperse hard spheres interacting through a screened Coulomb potential. Good agreement is noted when the volume fraction eta is adjusted during multivariate fitting of data, but the fitted value of eta is always lower than expected from the known Hb concentration of the samples. Calculations of cross-sections for polydisperse scatterers suggest that the samples may contain oligomers of the fundamental tetrameric Hb molecule.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briehl R. W., Ewert S. Effects of pH, 2,3-diphosphoglycerate and salts on gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1973 Nov 5;80(3):445–458. doi: 10.1016/0022-2836(73)90415-4. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Bendedouch D. Structure and interactions of proteins in solution studied by small-angle neutron scattering. Methods Enzymol. 1986;130:79–116. doi: 10.1016/0076-6879(86)30009-0. [DOI] [PubMed] [Google Scholar]
- Chien S., Usami S., Dellenback R. J., Bryant C. A. Comparative hemorheology--hematological implications of species differences in blood viscosity. Biorheology. 1971 Jun;8(1):35–57. doi: 10.3233/bir-1971-8106. [DOI] [PubMed] [Google Scholar]
- Griffith WL, Triolo R, Compere AL. Analytical scattering function of a polydisperse Percus-Yevick fluid with Schulz- ( Gamma -) distributed diameters. Phys Rev A Gen Phys. 1987 Mar 1;35(5):2200–2206. doi: 10.1103/physreva.35.2200. [DOI] [PubMed] [Google Scholar]
- Hansen J. P., Brown S. E., Sullivan R. J., Jr, Muhlbaier L. H. Factors related to an effective referral and consultation process. J Fam Pract. 1982 Oct;15(4):651–656. [PubMed] [Google Scholar]
- Johnson M. E., Danyluk S. S. Spin label detection of intermolecular interactions in carbonmonoxy sickle hemoglobin. Biophys J. 1978 Nov;24(2):517–524. doi: 10.1016/S0006-3495(78)85398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. R., Johnson C. S., Jr Photon correlation spectroscopy of hemoglobin: diffusion of oxy-HbA and oxy-HbS. Biopolymers. 1978 Jun;17(6):1581–1593. doi: 10.1002/bip.1978.360170615. [DOI] [PubMed] [Google Scholar]
- Keller K. H., Canales E. R., Yum S. I. Tracer and mutual diffusion coefficients of proteins. J Phys Chem. 1971 Feb 4;75(3):379–387. doi: 10.1021/j100673a015. [DOI] [PubMed] [Google Scholar]
- Krueger S., Nossal R. SANS studies of interacting hemoglobin in intact erythrocytes. Biophys J. 1988 Jan;53(1):97–105. doi: 10.1016/S0006-3495(88)83070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGattuta K. J., Sharma V. S., Nicoli D. F., Kothari B. K. Diffusion coefficients of hemoglobin by intensity fluctuation spectroscopy: effects of varying pH and ionic strength. Biophys J. 1981 Jan;33(1):63–79. doi: 10.1016/S0006-3495(81)84872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom T. R., Koenig S. H., Boussios T., Bertles J. F. Intermolecular interactions of oxygenated sickle hemoglobin molecules in cells and cell-free solutions. Biophys J. 1976 Jun;16(6):679–689. doi: 10.1016/S0006-3495(76)85721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. Thermodynamic nonideality and the dependence of partition coefficient upon solute concentration in exclusion chromatography. II. An improved theory of equilibrium partitioning of concentrated protein solutions. Application to hemoglobin. Biophys Chem. 1983 Sep;18(2):139–143. doi: 10.1016/0301-4622(83)85008-x. [DOI] [PubMed] [Google Scholar]
- Nishio I., Peetermans J., Tanaka T. Microscope laser light scattering spectroscopy of single biological cells. Cell Biophys. 1985 Jun;7(2):91–105. doi: 10.1007/BF02784485. [DOI] [PubMed] [Google Scholar]
- Nossal R., Glinka C. J., Chen S. H. SANS studies of concentrated protein solutions. I. Bovine serum albumin. Biopolymers. 1986 Jun;25(6):1157–1175. doi: 10.1002/bip.360250613. [DOI] [PubMed] [Google Scholar]
- Ray J., Englander S. W. Allosteric sensitivity in hemoglobin at the alpha-subunit N-terminus studied by hydrogen exchange. Biochemistry. 1986 May 20;25(10):3000–3007. doi: 10.1021/bi00358a040. [DOI] [PubMed] [Google Scholar]
- Riveros-Moreno V., Wittenberg J. B. The self-diffusion coefficients of myoglobin and hemoglobin in concentrated solutions. J Biol Chem. 1972 Feb 10;247(3):895–901. [PubMed] [Google Scholar]
- Ross P. D., Briehl R. W., Minton A. P. Temperature dependence of nonideality in concentrated solutions of hemoglobin. Biopolymers. 1978 Sep;17(9):2285–2288. doi: 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Analysis of non-ideal behavior in concentrated hemoglobin solutions. J Mol Biol. 1977 May 25;112(3):437–452. doi: 10.1016/s0022-2836(77)80191-5. [DOI] [PubMed] [Google Scholar]
- Schelten J., Schlecht P., Schmatz W., Mayer A. Neutron small angle scattering of hemoglobin. J Biol Chem. 1972 Sep 10;247(17):5436–5441. [PubMed] [Google Scholar]
- Tishler R. B., Carlson F. D. Quasi-elastic light scattering studies of membrane motion in single red blood cells. Biophys J. 1987 Jun;51(6):993–997. doi: 10.1016/S0006-3495(87)83428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr Concerted formation of the gel of hemoglobin S. Proc Natl Acad Sci U S A. 1973 May;70(5):1506–1508. doi: 10.1073/pnas.70.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]


