Abstract
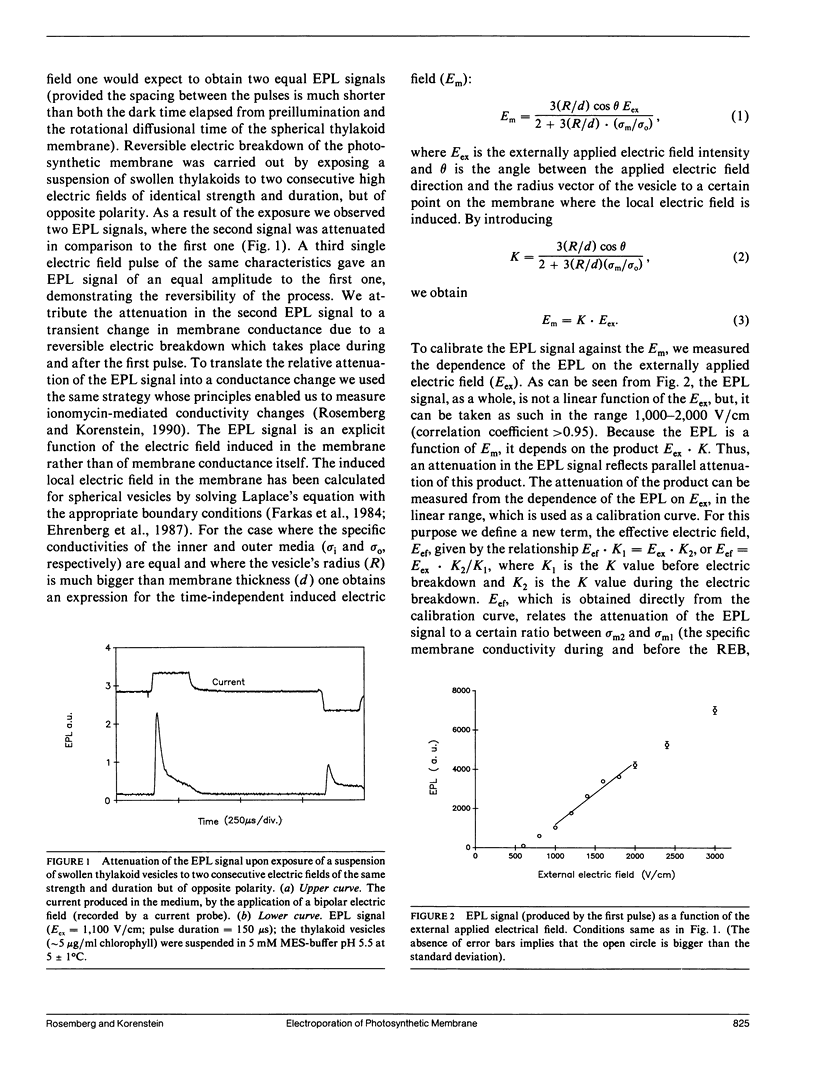
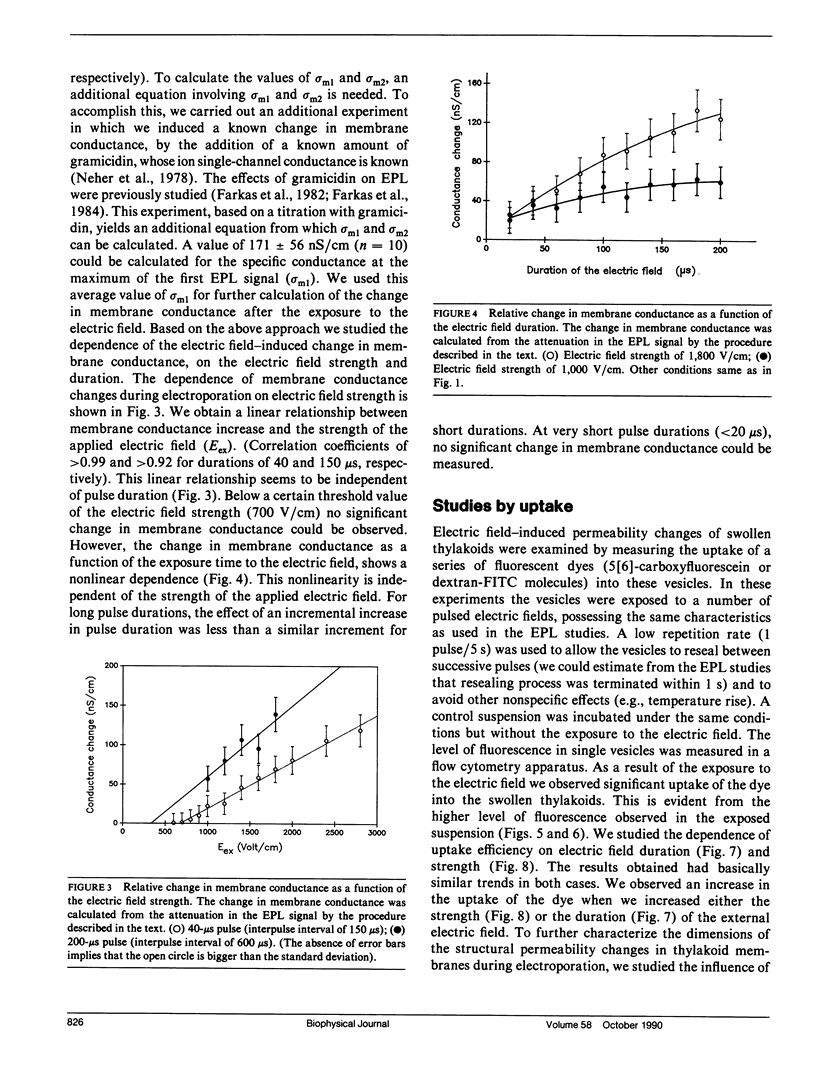
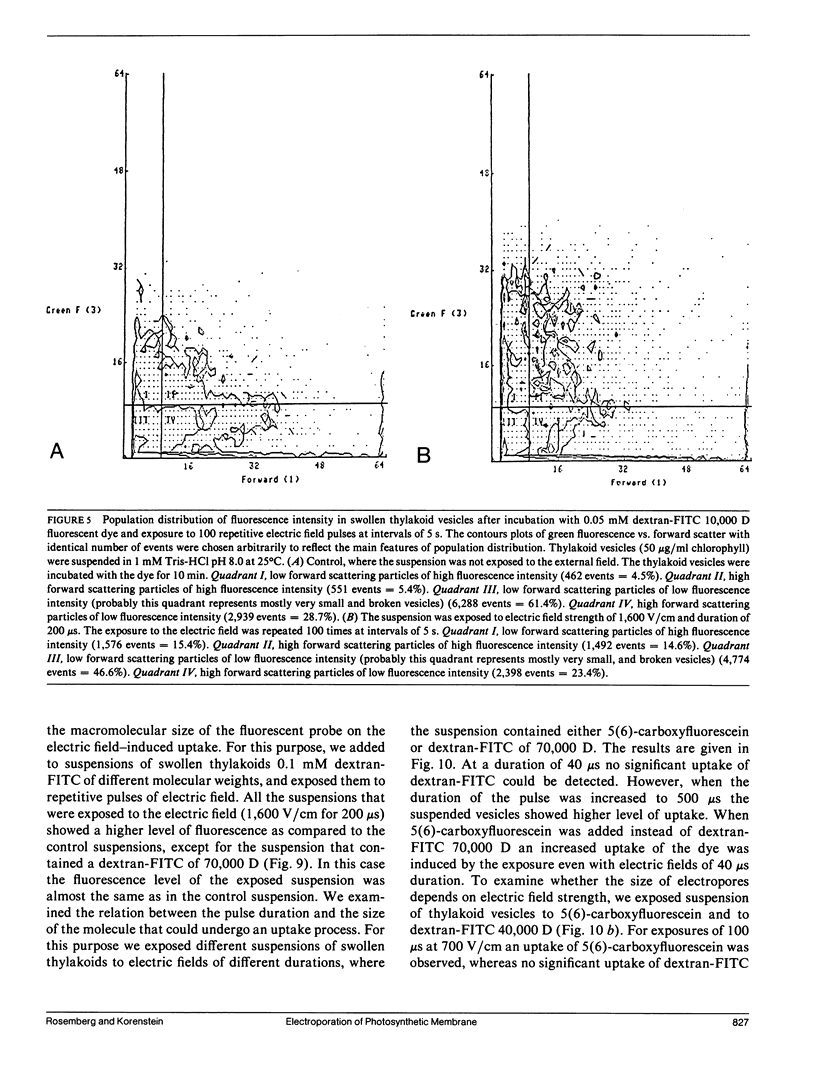
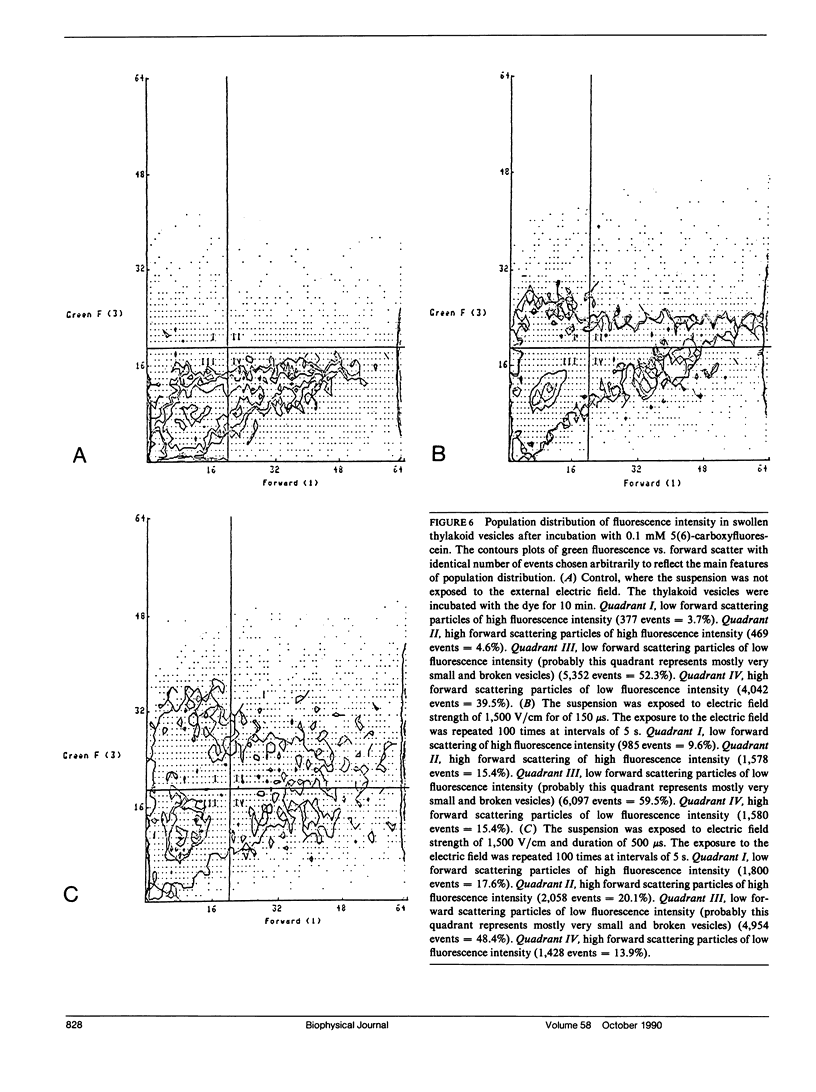
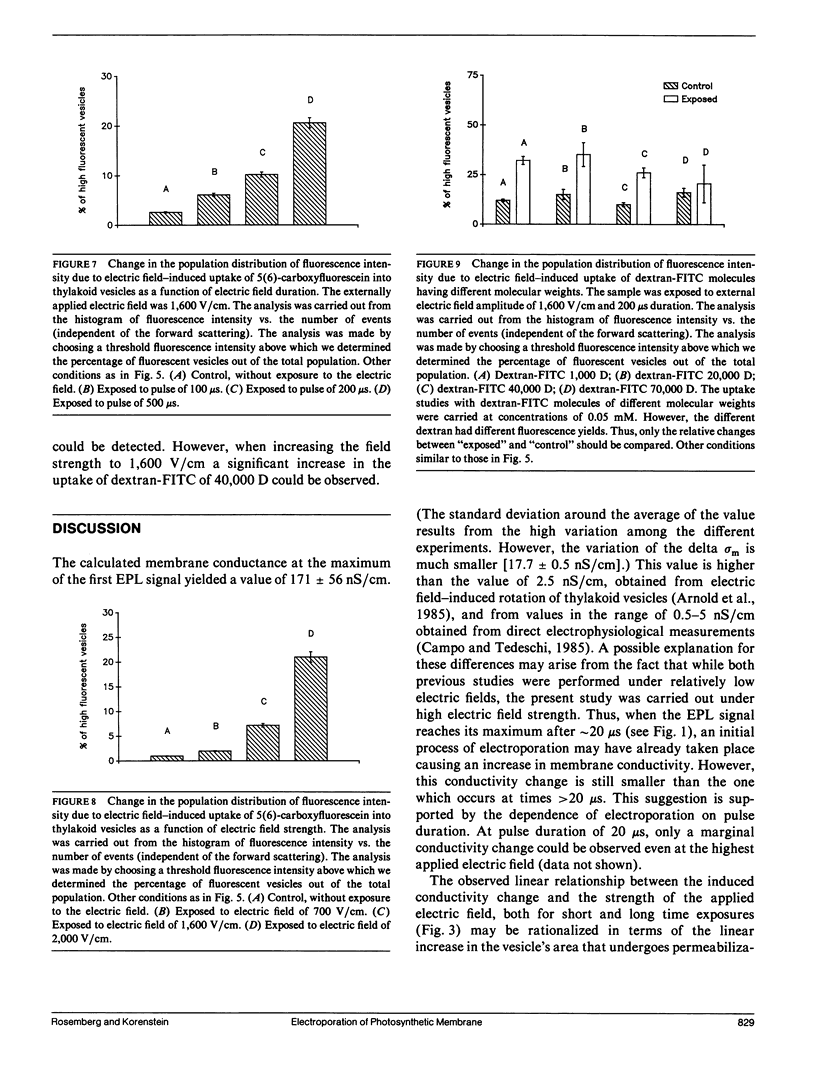
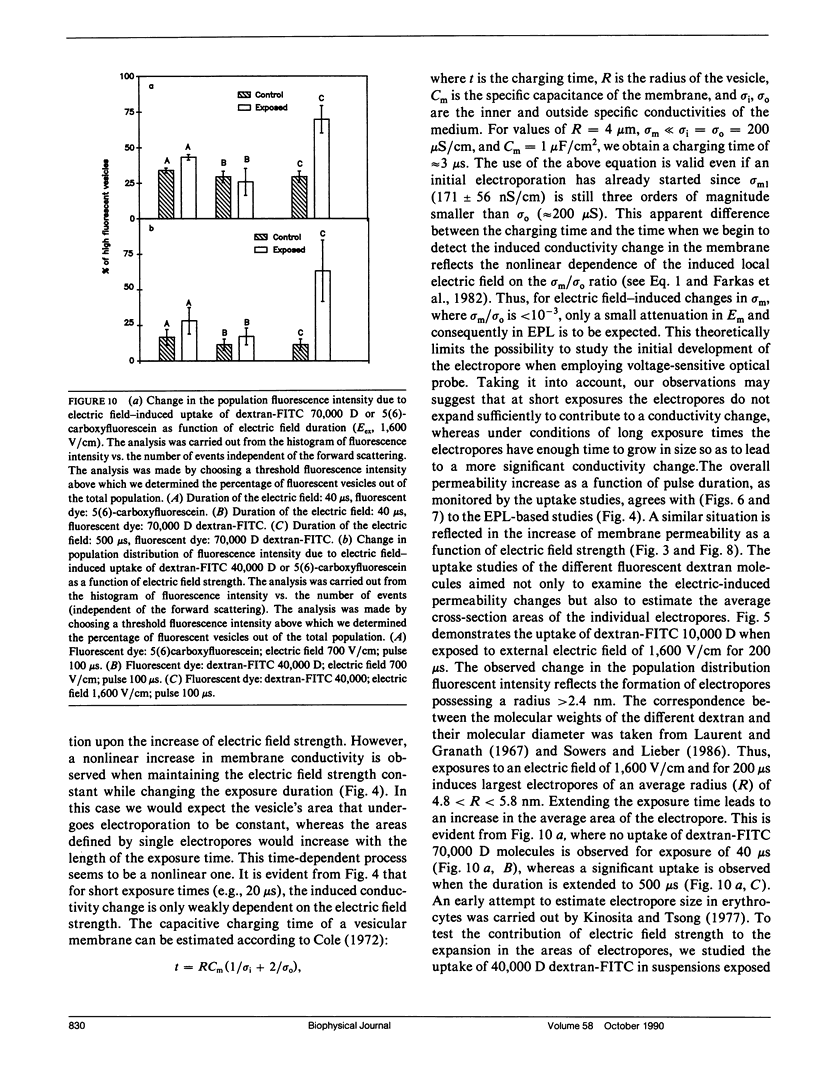
The study examines the relationship between electric field-induced conductivity and permeability changes in a biological membrane (electroporation) and the amplitude-duration parameters of the externally applied electric field. These reversible changes were characterized in giant photosynthetic membrane vesicles by means of the calibrated response of an intrinsic voltage-sensitive optical probe (electrophotoluminescence) and by the uptake studies of dextran-FITC fluorescent probes of different molecular weights. We quantitatively monitored electric field-induced conductivity changes by translating the electrophotoluminescence changes into conductivity changes. This was carried out by measuring the attenuation of the electrophotoluminescent signal after the addition of known amounts of gramicidin. The results demonstrate that electroporation involves the reversible formation of discrete holes in the membrane having radii <5.8 nm. The total area of the electric field-induced holes was 0.075% of the total surface of the vesicle. The formation of the electropores was affected differently by the electric field strength than by its duration. Increase in electric field strength caused increase in the total area of the vesicle that undergoes electroporation. Increase in the duration of the electric field increases the area of single electropores. Each of the two electric parameters can be rate limiting for the dynamics of electropore formation. These results are in accordance with the model of electroporation based on electric field-induced expansion of transient aqueous holes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Campo M. L., Tedeschi H. Protonmotive force and photophosphorylation in single swollen thylakoid vesicles. Eur J Biochem. 1985 Jun 18;149(3):511–516. doi: 10.1111/j.1432-1033.1985.tb08954.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B., Farkas D. L., Fluhler E. N., Lojewska Z., Loew L. M. Membrane potential induced by external electric field pulses can be followed with a potentiometric dye. Biophys J. 1987 May;51(5):833–837. doi: 10.1016/S0006-3495(87)83410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas D. L., Korenstein R., Malkin S. Electrophotoluminescence and the electrical properties of the photosynthetic membrane. I. Initial kinetics and the charging capacitance of the membrane. Biophys J. 1984 Feb;45(2):363–373. doi: 10.1016/S0006-3495(84)84160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J. W., Lockwood W. K., Collins F. S. Transfection of primary human skin fibroblasts by electroporation. Gene. 1988 Aug 15;68(1):167–172. doi: 10.1016/0378-1119(88)90610-5. [DOI] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Granath K. A. Fractionation of dextran and Ficoll by chromatography on Sephadex G-200. Biochim Biophys Acta. 1967 Mar 22;136(2):191–198. doi: 10.1016/0304-4165(67)90063-3. [DOI] [PubMed] [Google Scholar]
- Neher E., Sandblom J., Eisenman G. Ionic selectivity, saturation, and block in gramicidin A channels. II. Saturation behavior of single channel conductances and evidence for the existence of multiple binding sites in the channel. J Membr Biol. 1978 Apr 26;40(2):97–116. doi: 10.1007/BF01871143. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemberg Y., Korenstein R. A novel method for measuring membrane conductance changes by a voltage-sensitive optical probe. FEBS Lett. 1990 Apr 9;263(1):155–158. doi: 10.1016/0014-5793(90)80727-z. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Voltage modulation of membrane permeability and energy utilization in cells. Biosci Rep. 1983 Jun;3(6):487–505. doi: 10.1007/BF01120693. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Harrison G. I., Bliss J. G., Mourant J. R., Powell K. T. Electroporation: high frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988 Feb 29;229(1):30–34. doi: 10.1016/0014-5793(88)80791-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electric field-mediated fusion and related electrical phenomena. Biochim Biophys Acta. 1982 Nov 30;694(3):227–277. doi: 10.1016/0304-4157(82)90007-7. [DOI] [PubMed] [Google Scholar]


