Abstract
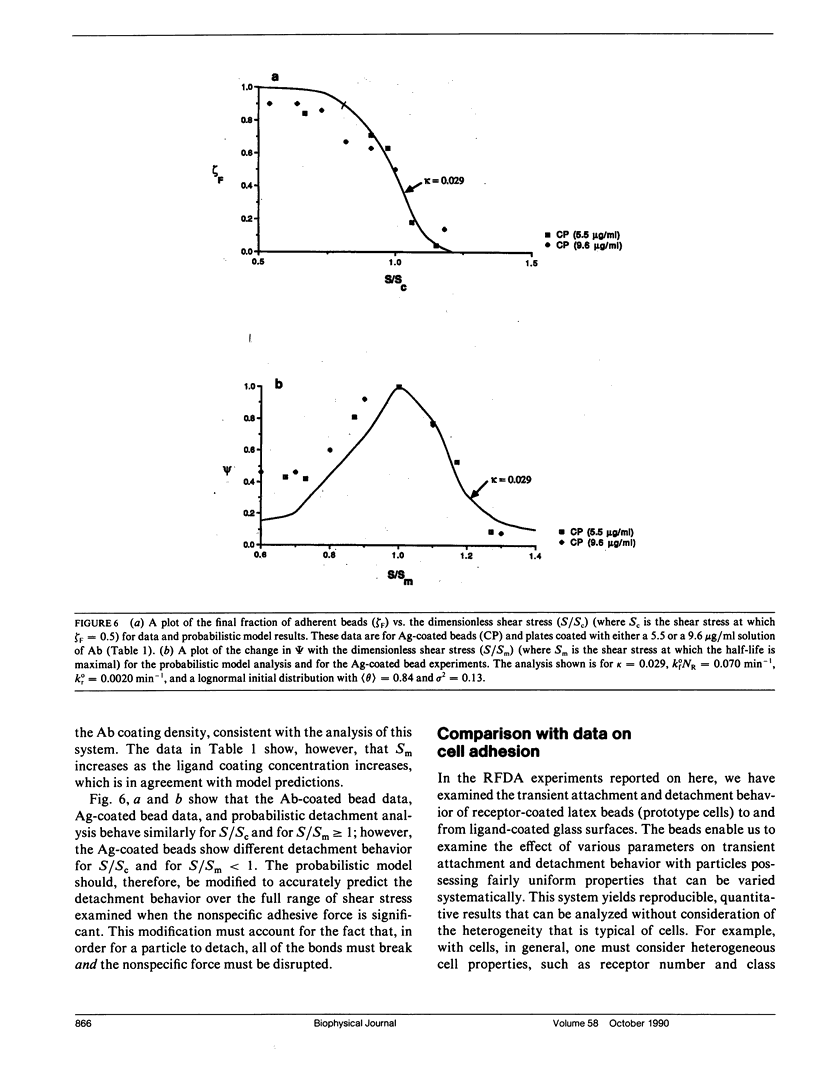
Quantitative information regarding the kinetics of receptor-mediated cell adhesion to a ligand-coated surface are crucial for understanding the role of certain key parameters in many physiological and biotechnology-related processes. Here, we use the probabilistic attachment and detachment models developed in the preceding paper to interpret transient data from well-defined experiments. These data are obtained with a simple model cell system that consists of receptor-coated latex beads (prototype cells) and a Radial-Flow Detachment Assay (RFDA) using a ligand-coated glass disc. The receptors and ligands used in this work are complementary antibodies. The beads enable us to examine transient behavior with particles that possess fairly uniform properties that can be varied systematically, and the RFDA is designed for direct observation of adhesion to the ligand-coated glass surface over a range of shear stresses. Our experiments focus on the effects of surface shear stress, receptor density, and ligand density. These data provide a crucial test of the probabilistic framework. We show that these data can be explained with the probabilistic analyses, whereas they cannot be readily interpreted on the basis of a deterministic analysis. In addition, we examine transient data on cell adhesion reported from other assays, demonstrating the consistency of these data with the predictions of the probabilistic models.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHOLOMAY A. F. A stochastic approach to statistical kinetics with application to enzyme kinetics. Biochemistry. 1962 Mar;1:223–230. doi: 10.1021/bi00908a005. [DOI] [PubMed] [Google Scholar]
- Basch R. S., Berman J. W., Lakow E. Cell separation using positive immunoselective techniques. J Immunol Methods. 1983 Feb 11;56(3):269–280. doi: 10.1016/s0022-1759(83)80016-7. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Kalamasz D., Martin P. Elimination of Daudi lymphoblasts from human bone marrow using avidin-biotin immunoadsorption. Blood. 1986 Feb;67(2):509–515. [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Kalamasz D. Positive selection of viable cell populations using avidin-biotin immunoadsorption. J Immunol Methods. 1986 Jul 11;91(1):11–19. doi: 10.1016/0022-1759(86)90096-7. [DOI] [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Kalamasz D., Schuening F., Deeg H. J., Graham T., Storb R. Engraftment of dogs with Ia-positive marrow cells isolated by avidin-biotin immunoadsorption. Blood. 1987 May;69(5):1363–1367. [PubMed] [Google Scholar]
- Berenson R. J., Levitt L. J., Levy R., Miller R. A. Cellular immunoabsorption using monoclonal antibodies. Selective removal of T cells from peripheral blood and bone marrow. Transplantation. 1984 Aug;38(2):136–142. [PubMed] [Google Scholar]
- Bongrand P., Capo C., Benoliel A. M., Depieds R. Evaluation of intercellular adhesion with a very simple technique. J Immunol Methods. 1979;28(1-2):133–141. doi: 10.1016/0022-1759(79)90335-1. [DOI] [PubMed] [Google Scholar]
- Capo C., Garrouste F., Benoliel A. M., Bongrand P., Ryter A., Bell G. I. Concanavalin-A-mediated thymocyte agglutination: a model for a quantitative study of cell adhesion. J Cell Sci. 1982 Aug;56:21–48. doi: 10.1242/jcs.56.1.21. [DOI] [PubMed] [Google Scholar]
- Cozens-Roberts C., Lauffenburger D. A., Quinn J. A. Receptor-mediated cell attachment and detachment kinetics. I. Probabilistic model and analysis. Biophys J. 1990 Oct;58(4):841–856. doi: 10.1016/S0006-3495(90)82430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenberger D. A. Receptor-mediated adhesion phenomena. Model studies with the Radical-Flow Detachment Assay. Biophys J. 1990 Jul;58(1):107–125. doi: 10.1016/S0006-3495(90)82357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C. The biophysics of ligand-receptor interactions. Q Rev Biophys. 1980 May;13(2):201–230. doi: 10.1017/s0033583500001657. [DOI] [PubMed] [Google Scholar]
- Dejana E., Colella S., Languino L. R., Balconi G., Corbascio G. C., Marchisio P. C. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987 May;104(5):1403–1411. doi: 10.1083/jcb.104.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin S. G., Ives C. L., McIntire L. V., Navarro L. T. Response of cultured endothelial cells to steady flow. Microvasc Res. 1984 Jul;28(1):87–94. doi: 10.1016/0026-2862(84)90031-1. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., von Tscharner V., McConnell H. M. Specific antibody-dependent interactions between macrophages and lipid haptens in planar lipid monolayers. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4552–4556. doi: 10.1073/pnas.78.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Lauffenburger D. A. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987 Sep;52(3):475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Hornick C. L., Karush F. The interaction of hapten-coupled bacteriophage phi-X-174 with antihapten antibody. Isr J Med Sci. 1969 Mar-Apr;5(2):163–170. [PubMed] [Google Scholar]
- Jarrell B. E., Williams S. K., Solomon L., Speicher L., Koolpe E., Radomski J., Carabasi R. A., Greener D., Rosato F. E. Use of an endothelial monolayer on a vascular graft prior to implantation. Temporal dynamics and compatibility with the operating room. Ann Surg. 1986 Jun;203(6):671–678. doi: 10.1097/00000658-198606000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler K. A., Herring M. B., Arnold M. P., Glover J. L., Park H. M., Helmus M. N., Bendick P. J. Enhanced strength of endothelial attachment on polyester elastomer and polytetrafluoroethylene graft surfaces with fibronectin substrate. J Vasc Surg. 1986 Jan;3(1):58–64. doi: 10.1067/mva.1986.avs0030058. [DOI] [PubMed] [Google Scholar]
- Kinzel V., Richards J., Kübler D. Lectin receptor sites at the cell surface employed for affinity separation of tissue culture cells. Basic requirements as realized by lens culinaris lectin (LCL) immobilized on 2tb-sepharose. Exp Cell Res. 1977 Mar 15;105(2):389–400. doi: 10.1016/0014-4827(77)90136-7. [DOI] [PubMed] [Google Scholar]
- Kvalheim G., Fjeld J. G., Pihl A., Funderud S., Ugelstad J., Fodstad O., Nustad K. Immunomagnetic removal of B-lymphoma cells using a novel mono-sized magnetizable polymer bead, M-280, in conjunction with primary IgM and IgG antibodies. Bone Marrow Transplant. 1989 Sep;4(5):567–574. [PubMed] [Google Scholar]
- Kvalheim G., Fodstad O., Pihl A., Nustad K., Pharo A., Ugelstad J., Funderud S. Elimination of B-lymphoma cells from human bone marrow: model experiments using monodisperse magnetic particles coated with primary monoclonal antibodies. Cancer Res. 1987 Feb 1;47(3):846–851. [PubMed] [Google Scholar]
- Lauffenburger D., DeLisi C. Cell surface receptors: physical chemistry and cellular regulation. Int Rev Cytol. 1983;84:269–302. doi: 10.1016/s0074-7696(08)61020-7. [DOI] [PubMed] [Google Scholar]
- Liao N. S., St John J., Du Z. J., Cheung H. T. Adhesion of lymphoid cell lines to fibronectin-coated substratum: biochemical and physiological characterization and the identification of a 140-kDa fibronectin receptor. Exp Cell Res. 1987 Aug;171(2):306–320. doi: 10.1016/0014-4827(87)90164-9. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Giblett E. R., Hansen J. A. Phenotyping human leukemic T-cell lines: enzyme markers, surface antigens, and cytogenetics. Immunogenetics. 1982;15(4):385–398. doi: 10.1007/BF00364262. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Wessel G. M., Marchase R. B. Intercellular recognition: quantitation of initial binding events. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4975–4979. doi: 10.1073/pnas.78.8.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege J. L., Capo C., Benoliel A. M., Bongrand P. Determination of binding strength and kinetics of binding initiation. A model study made on the adhesive properties of P388D1 macrophage-like cells. Cell Biophys. 1986 Apr;8(2):141–160. doi: 10.1007/BF02788478. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., PRESSMAN D. Heterogeneity and average combining constants of antibodies from individual rabbits. J Immunol. 1958 Jun;80(6):417–428. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D., Battye F. L. Separation of mouse bone marrow cells using wheat germ agglutinin affinity chromatographyy. Aust J Exp Biol Med Sci. 1978 Dec;56(6):663–679. doi: 10.1038/icb.1978.74. [DOI] [PubMed] [Google Scholar]
- Nygren H., Kaartinen M., Stenberg M. Determination by ellipsometry of the affinity of monoclonal antibodies. J Immunol Methods. 1986 Sep 27;92(2):219–225. doi: 10.1016/0022-1759(86)90169-9. [DOI] [PubMed] [Google Scholar]
- Pecht I., Lancet D. Kinetics of antibody-hapten interactions. Mol Biol Biochem Biophys. 1977;24:306–338. doi: 10.1007/978-3-642-81117-3_9. [DOI] [PubMed] [Google Scholar]
- Perelson A. S., Goldstein B., Rocklin S. Optimal strategies in immunology III. The IgM-IgG switch. J Math Biol. 1980 Nov;10(3):209–256. doi: 10.1007/BF00276984. [DOI] [PubMed] [Google Scholar]
- Pratt B. M., Form D., Madri J. A. Endothelial cell-extracellular matrix interactions. Ann N Y Acad Sci. 1985;460:274–288. doi: 10.1111/j.1749-6632.1985.tb51175.x. [DOI] [PubMed] [Google Scholar]
- Pratt K. J., Jarrell B. E., Williams S. K., Carabasi R. A., Rupnick M. A., Hubbard F. A. Kinetics of endothelial cell-surface attachment forces. J Vasc Surg. 1988 Apr;7(4):591–599. [PubMed] [Google Scholar]
- Ramalanjaona G., Kempczinski R. F., Rosenman J. E., Douville E. C., Silberstein E. B. The effect of fibronectin coating on endothelial cell kinetics in polytetrafluoroethylene grafts. J Vasc Surg. 1986 Feb;3(2):264–272. doi: 10.1067/mva.1986.avs0030264. [DOI] [PubMed] [Google Scholar]
- Rosenman J. E., Kempczinski R. F., Pearce W. H., Silberstein E. B. Kinetics of endothelial cell seeding. J Vasc Surg. 1985 Nov;2(6):778–784. [PubMed] [Google Scholar]
- Ruckenstein E. Dynamics of cell deposition on surfaces. J Theor Biol. 1975 Jun;51(2):429–438. doi: 10.1016/0022-5193(75)90072-7. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Sachs L. Receptor mobility and the binding of cells to lectin-coated fibers. J Cell Biol. 1975 Jul;66(1):76–85. doi: 10.1083/jcb.66.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger R. C., Vo D. D., Ugelstad J., Reynolds C. P. Removal of neuroblastoma cells from bone marrow with monoclonal antibodies and magnetic immunobeads. Prog Clin Biol Res. 1986;211:285–293. [PubMed] [Google Scholar]
- Stenberg M., Stiblert L., Nygren H. External diffusion in solid-phase immunoassays. J Theor Biol. 1986 May 21;120(2):129–140. doi: 10.1016/s0022-5193(86)80169-2. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Sung L. A., Crimmins M., Burakoff S. J., Chien S. Determination of junction avidity of cytolytic T cell and target cell. Science. 1986 Dec 12;234(4782):1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- Umbreit J., Roseman S. A requirement for reversible binding between aggregating embryonic cells before stable adhesion. J Biol Chem. 1975 Dec 25;250(24):9360–9368. [PubMed] [Google Scholar]
- Weigel P. H., Schnaar R. L., Kuhlenschmidt M. S., Schmell E., Lee R. T., Lee Y. C., Roseman S. Adhesion of hepatocytes to immobilized sugars. A threshold phenomenon. J Biol Chem. 1979 Nov 10;254(21):10830–10838. [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M., Forrester J. V., Dunn G. A. Chemokinetic accumulation of human neutrophils on immune complex-coated substrata: analysis at a boundary. J Cell Biol. 1984 Nov;99(5):1761–1768. doi: 10.1083/jcb.99.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]



