Abstract
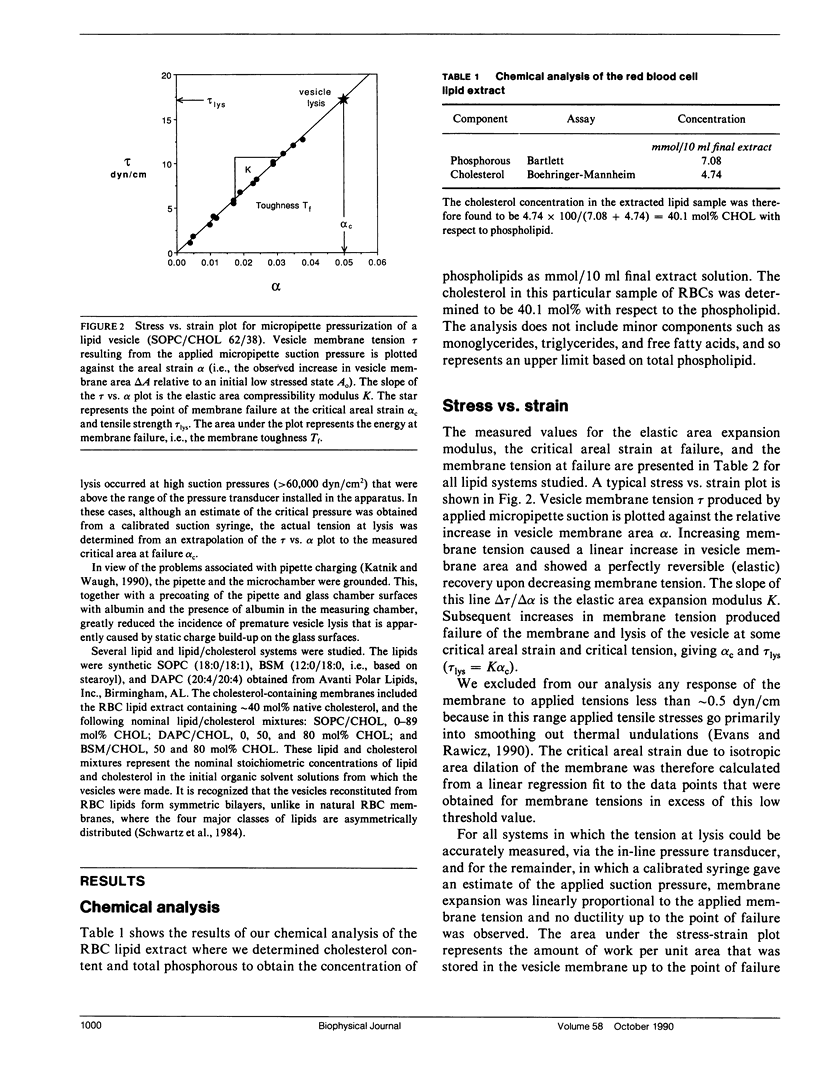
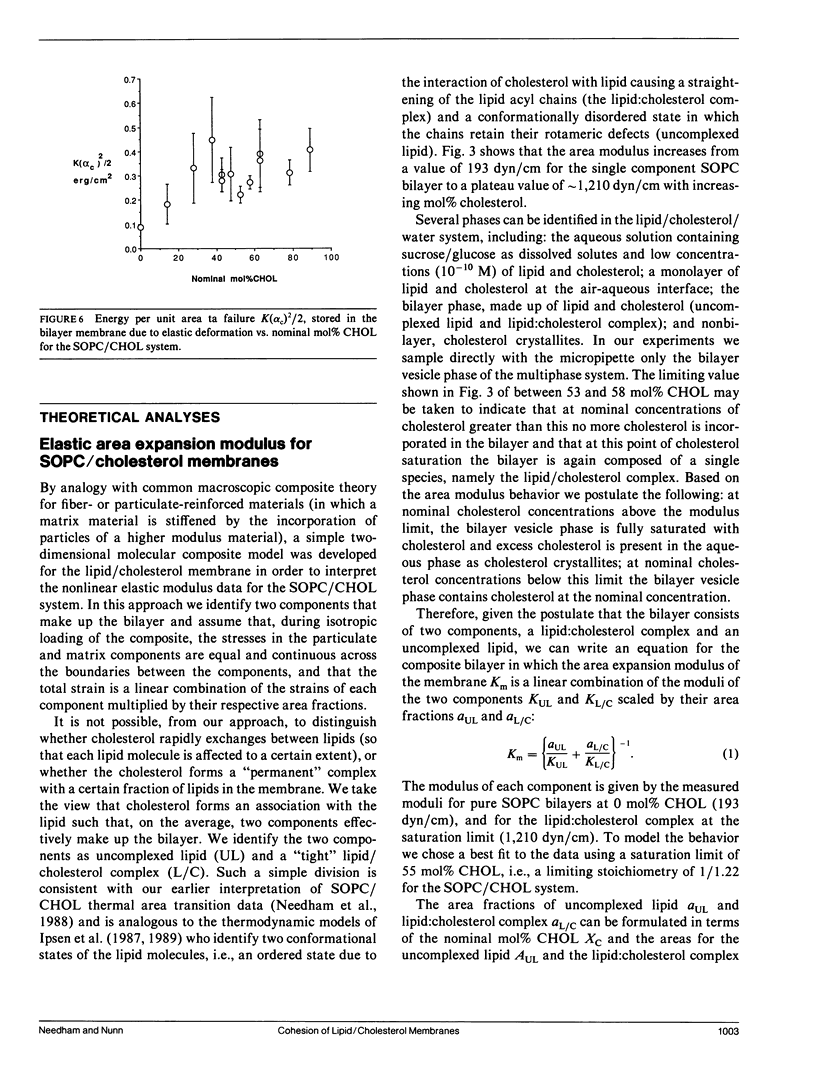
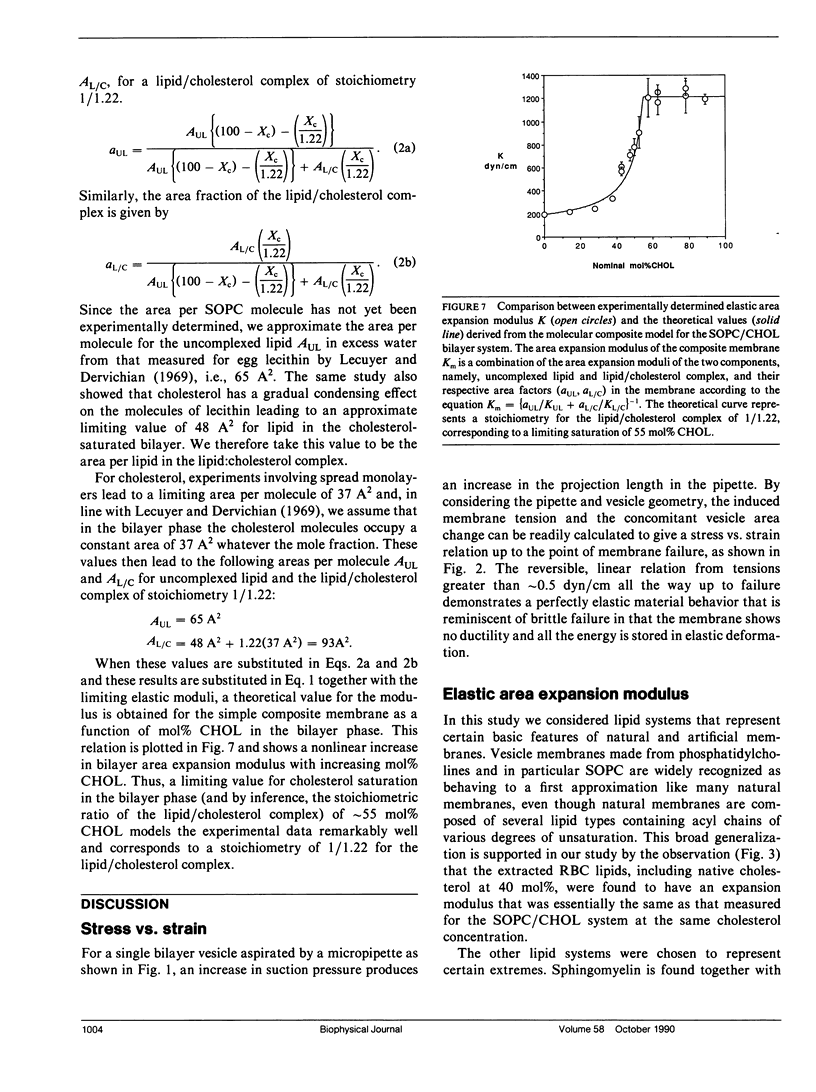
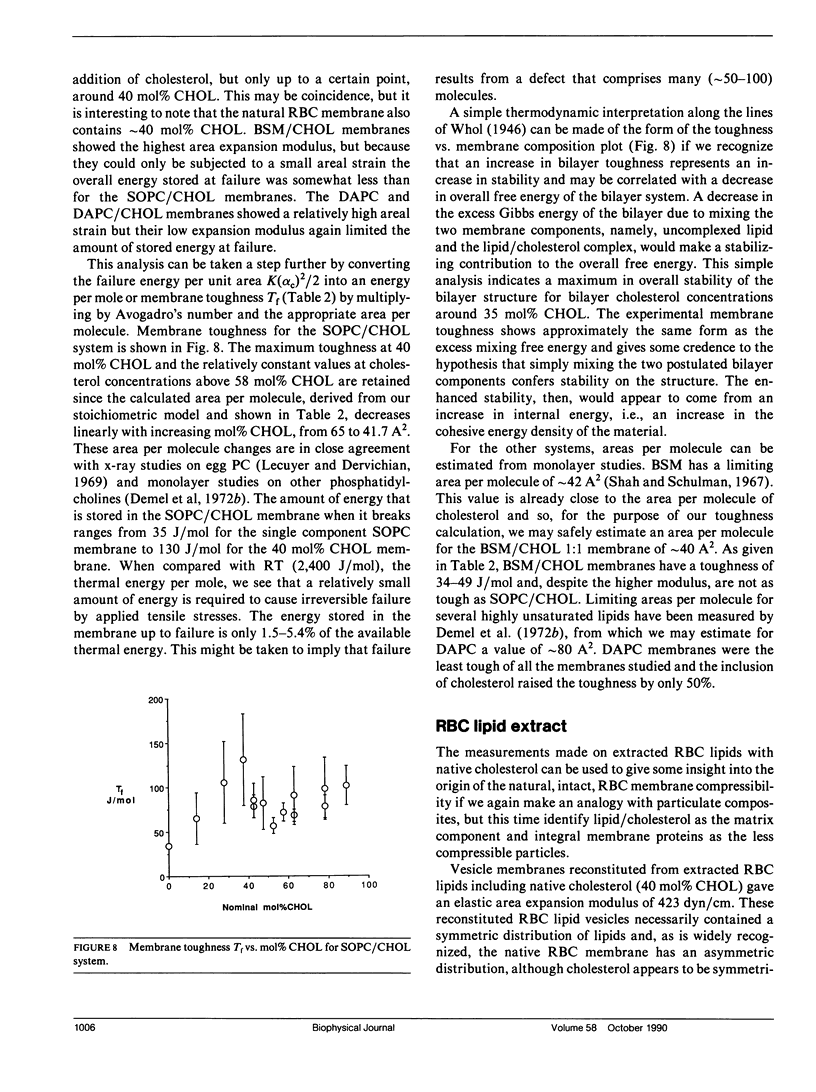
Giant bilayer vesicles were reconstituted from several lipids and lipid/cholesterol (CHOL) mixtures: stearolyloleoylphosphatidylcholine (SOPC), bovine sphingomyelin (BSM), diarachidonylphosphatidylcholine (DAPC), SOPC/CHOL, BSM/CHOL, DAPC/CHOL, and extracted red blood cell (RBC) lipids with native cholesterol. Single-walled vesicles were manipulated by micropipette suction and several membrane material properties were determined. The properties measured were the elastic area compressibility modulus K, the critical areal strain alpha c, and the tensile strength tau lys, from which the failure energy or membrane toughness Tf was calculated. The elastic area expansion moduli for these lipid and lipid/cholesterol bilayers ranged from 57 dyn/cm for DAPC to 1,734 dyn/cm for BSM/CHOL. The SOPC/CHOL series and RBC lipids had intermediate values. The results indicated that the presence of cholesterol is the single most influential factor in increasing bilayer cohesion, but only for lipids where both chains are saturated, or mono- or diunsaturated. Multiple unsaturation in both lipid chains inhibits the condensing effect of cholesterol in bilayers. The SOPC/CHOL system was studied in more detail. The area expansion modulus showed a nonlinear increase with increasing cholesterol concentration up to a constant plateau, indicating a saturation limit for cholesterol in the bilayer phase of approximately 55 mol% CHOL. The membrane compressibility was modeled by a property-averaging composite theory involving two bilayer components, namely, uncomplexed lipid and a lipid/cholesterol complex of stoichiometry 1/1.22. The area expansion modulus of this molecular composite membrane was evaluated by a combination of the expansion moduli of each component scaled by their area fractions in the bilayer. Bilayer toughness, which is the energy stored in the bilayer at failure, showed a maximum value at approximately 40 mol% CHOL. This breakdown energy was found to be only a fraction of the available thermal energy, implying that many molecules (approximately 50-100) may be involved in forming the defect structure that leads to failure. The area expansion modulus of extracted RBC lipids with native cholesterol was compared with recent measurements of intact RBC membrane compressibility. The natural membrane was also modeled as a simple composite made up to a compressible lipid/cholesterol matrix containing relatively incompressible transmembrane proteins. It appears that the interaction of incompressible proteins with surrounding lipid confers enhanced compressibility on the composite structure.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Drenthe E. H., Klompmakers A. A., Bonting S. L., Daemen F. J. Transbilayer distribution of phospholipids in photoreceptor membrane studied with trinitrobenzenesulfonate alone and in combination with phospholipase D. Biochim Biophys Acta. 1980 Dec 2;603(1):130–141. doi: 10.1016/0005-2736(80)90396-x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Haydon D. A. Water permeability of lipid membranes. Physiol Rev. 1980 Apr;60(2):510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- Guyer W., Bloch K. Phosphatidylcholine and cholesterol interactions in model membranes. Chem Phys Lipids. 1983 Nov;33(4):313–322. doi: 10.1016/0009-3084(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Karlström G., Mouritsen O. G., Wennerström H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987 Nov 27;905(1):162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Zuckermann M. J. Theory of thermal anomalies in the specific heat of lipid bilayers containing cholesterol. Biophys J. 1989 Oct;56(4):661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katnik C., Waugh R. Alterations of the apparent area expansivity modulus of red blood cell membrane by electric fields. Biophys J. 1990 Apr;57(4):877–882. doi: 10.1016/S0006-3495(90)82607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll W., Schmidt G., Ibel K., Sackmann E. Small-angle neutron scattering study of lateral phase separation in dimyristoylphosphatidylcholine-cholesterol mixed membranes. Biochemistry. 1985 Sep 10;24(19):5240–5246. doi: 10.1021/bi00340a043. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lange Y., Dolde J., Steck T. L. The rate of transmembrane movement of cholesterol in the human erythrocyte. J Biol Chem. 1981 Jun 10;256(11):5321–5323. [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Li L. K., So L., Spector A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J Lipid Res. 1985 May;26(5):600–609. [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Needham D., Evans E. Structure and mechanical properties of giant lipid (DMPC) vesicle bilayers from 20 degrees C below to 10 degrees C above the liquid crystal-crystalline phase transition at 24 degrees C. Biochemistry. 1988 Oct 18;27(21):8261–8269. doi: 10.1021/bi00421a041. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys J. 1989 May;55(5):1001–1009. doi: 10.1016/S0006-3495(89)82898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., McIntosh T. J., Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 1988 Jun 28;27(13):4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Presti F. T., Pace R. J., Chan S. I. Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry. 1982 Aug 3;21(16):3831–3835. doi: 10.1021/bi00259a017. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Chiu D. T., Lubin B. Studies on the organization of plasma membrane phospholipids in human erythrocytes. Prog Clin Biol Res. 1984;159:89–122. [PubMed] [Google Scholar]
- Scott H. L., Kalaskar S. Lipid chains and cholesterol in model membranes: a Monte Carlo Study. Biochemistry. 1989 May 2;28(9):3687–3691. doi: 10.1021/bi00435a010. [DOI] [PubMed] [Google Scholar]
- Shah D. O., Schulman J. H. Interaction of calcium ions with lecithin and sphingomyelin monolayers. Lipids. 1967 Jan;2(1):21–27. doi: 10.1007/BF02531995. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- Winterhalter M, Helfrich W. Effect of voltage on pores in membranes. Phys Rev A Gen Phys. 1987 Dec 15;36(12):5874–5876. doi: 10.1103/physreva.36.5874. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Martin R. B., Lala A. K., Lin H. K., Bloch K. Differential effects of cholesterol and lanosterol on artificial membranes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4924–4926. doi: 10.1073/pnas.74.11.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]



