Abstract
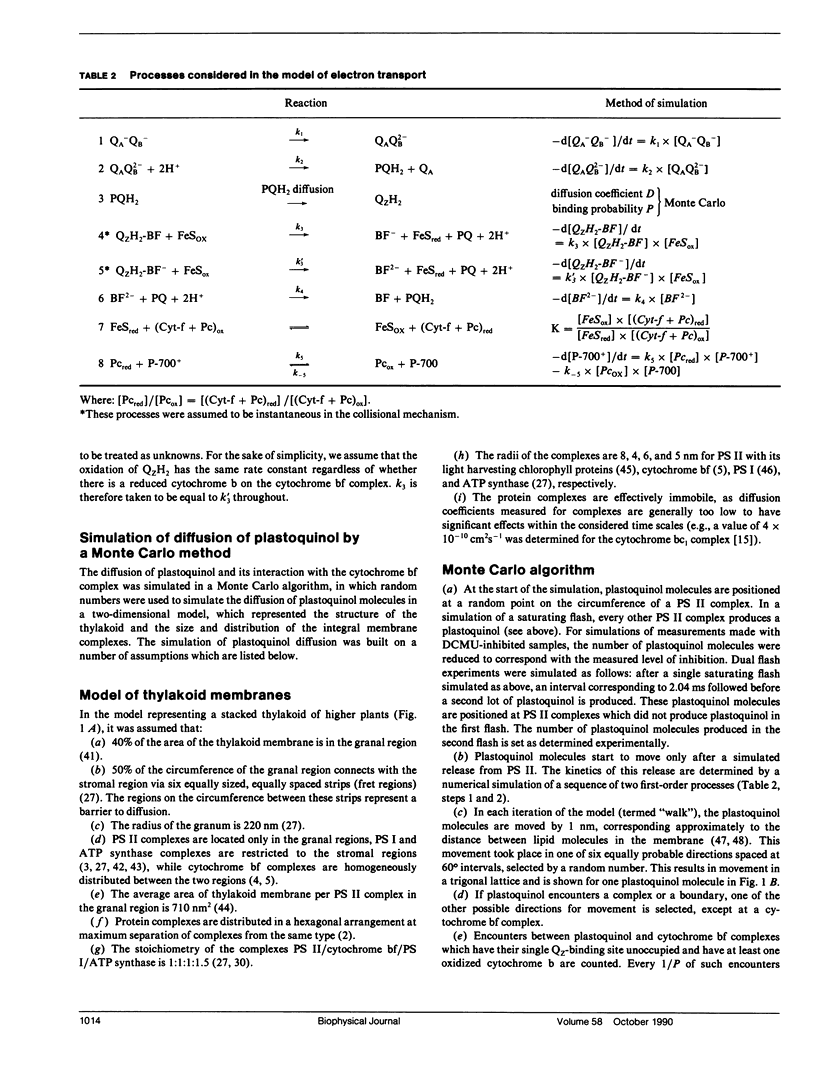
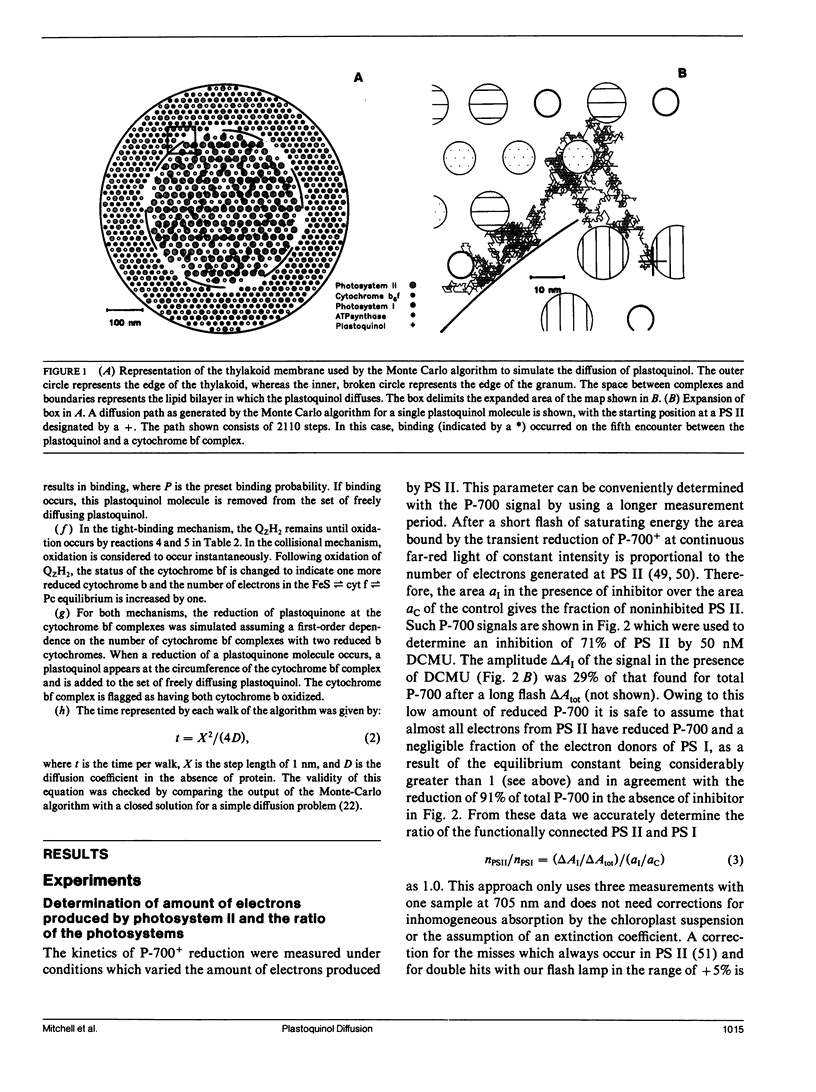
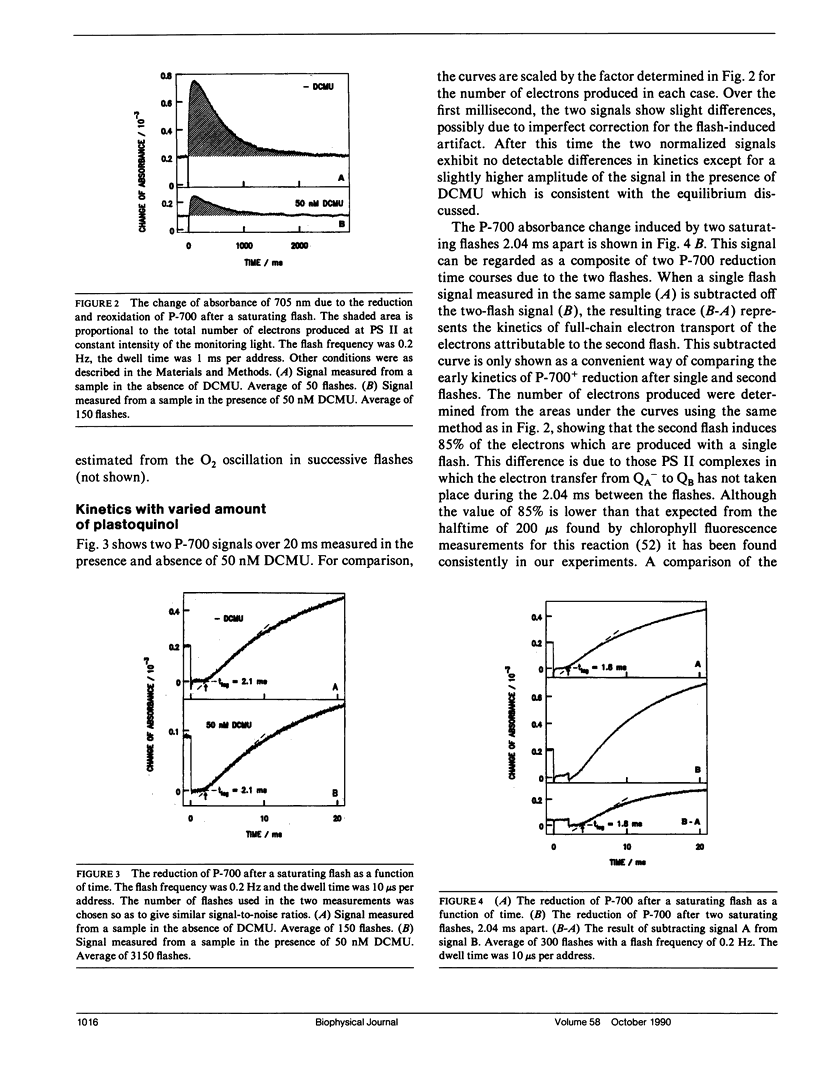
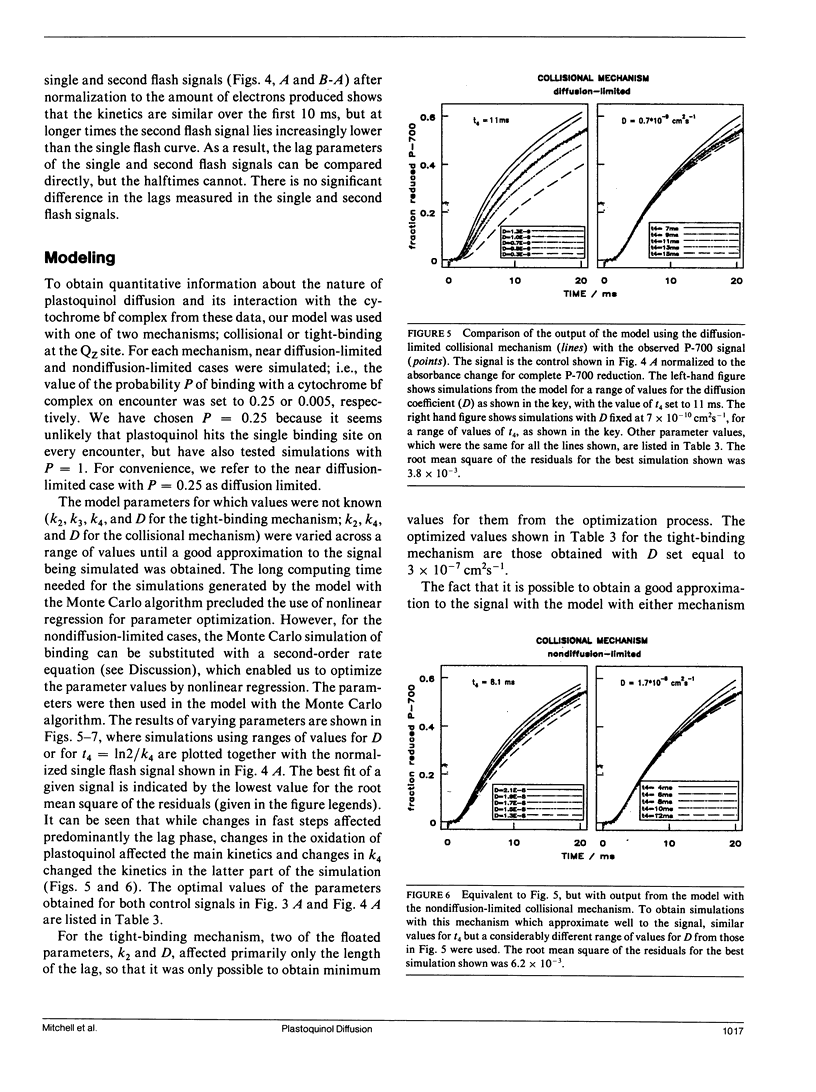
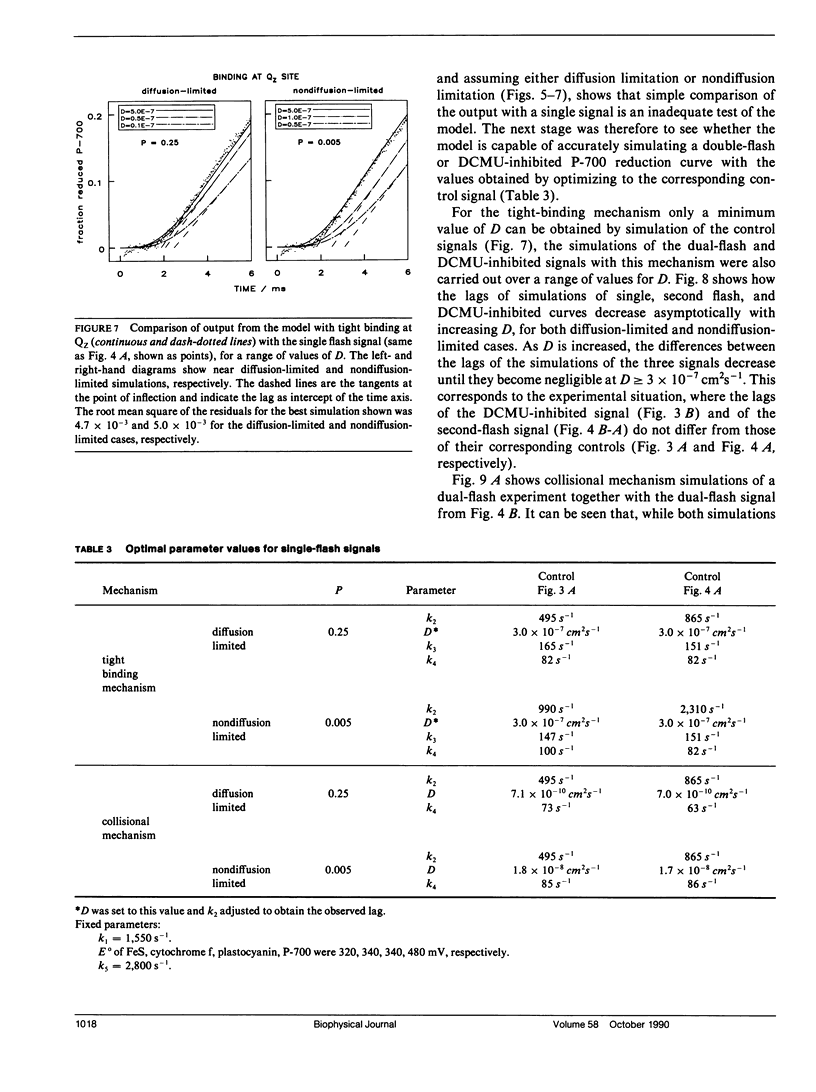
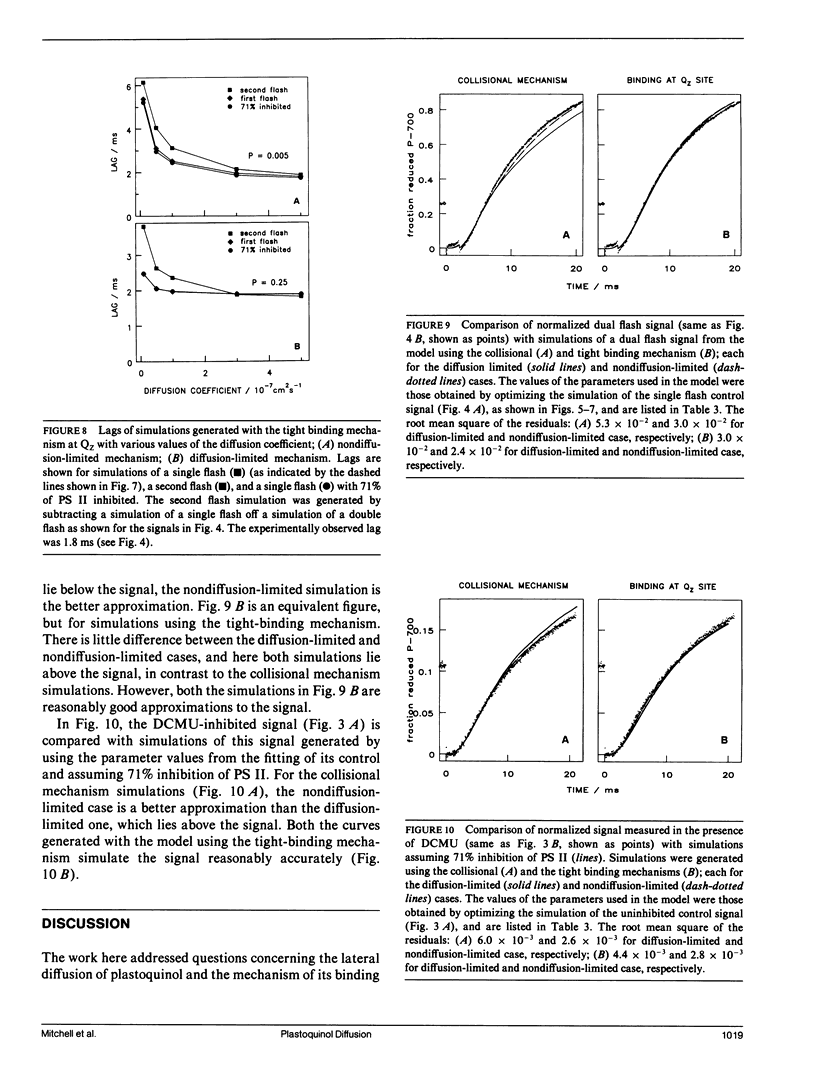
The diffusion of plastoquinol and its binding to the cytochrome bf complex, which occurs during linear photosynthetic electron transport and is analogous to reaction sequences found in most energy-converting membranes, has been studied in intact thylakoid membranes. The flash-induced electron transfer between the laterally separated photosystems II and photosystems I was measured by following the sigmoidal reduction kinetics of P-700+ after previous oxidation of the intersystem electron carriers. The amount of flash-induced plastoquinol produced at photosystem II was (a) reduced by inhibition with dichlorophenyl-dimethylurea and (b) increased by giving a second saturating flash. These signals were simulated by a new model which combines a deterministic simulation of reaction kinetics with a Monte Carlo approach to the diffusion of plastoquinol, taking into account the known structural features of the thylakoid membrane. The plastoquinol molecules were assumed to be oxidized by either a diffusion-limited or a nondiffusion-limited step in a collisional mechanism or after binding to the cytochrome bf complex. The model was able to account for the experimental observations with a nondiffusion-limited collisional mechanism or with a binding mechanism, giving minimum values for the diffusion coefficient of plastoquinol of 2 × 10-8 cm2s-1 and 3 × 10-7 cm2s-1, respectively.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., Staehelin L. A. Spatial organization of the cytochrome b6-f complex within chloroplast thylakoid membranes. Biochim Biophys Acta. 1986 Apr 2;849(1):94–103. doi: 10.1016/0005-2728(86)90100-3. [DOI] [PubMed] [Google Scholar]
- Andersson B., Anderson J. M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):427–440. doi: 10.1016/0005-2728(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Zara S. J., Barber J. A method for estimating lateral diffusion coefficients in membranes from steady-state fluorescence quenching studies. Biophys J. 1987 May;51(5):735–744. doi: 10.1016/S0006-3495(87)83400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohner H., Böhme H., Böger P. Reciprocal formation of plastocyanin and cytochrome c-553 and the influence of cupric ions on photosynthetic electron transport. Biochim Biophys Acta. 1980 Aug 5;592(1):103–112. doi: 10.1016/0005-2728(80)90117-6. [DOI] [PubMed] [Google Scholar]
- Bowes J. M., Crofts A. R. Binary oscillations in the rate of reoxidation of the primary acceptor of photosystem II. Biochim Biophys Acta. 1980 May 9;590(3):373–384. doi: 10.1016/0005-2728(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. Lateral diffusion as a rate-limiting step in ubiquinone-mediated mitochondrial electron transport. J Biol Chem. 1989 Mar 25;264(9):4978–4985. [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988 Oct 5;263(28):14359–14367. [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fato R., Battino M., Degli Esposti M., Parenti Castelli G., Lenaz G. Determination of partition and lateral diffusion coefficients of ubiquinones by fluorescence quenching of n-(9-anthroyloxy)stearic acids in phospholipid vesicles and mitochondrial membranes. Biochemistry. 1986 Jun 3;25(11):3378–3390. doi: 10.1021/bi00359a043. [DOI] [PubMed] [Google Scholar]
- Fato R., Battino M., Parenti Castelli G., Lenaz G. Measurement of the lateral diffusion coefficients of ubiquinones in lipid vesicles by fluorescence quenching of 12-(9-anthroyl)stearate. FEBS Lett. 1985 Jan 7;179(2):238–242. doi: 10.1016/0014-5793(85)80526-3. [DOI] [PubMed] [Google Scholar]
- Gupte S., Wu E. S., Hoechli L., Hoechli M., Jacobson K., Sowers A. E., Hackenbrock C. R. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proc Natl Acad Sci U S A. 1984 May;81(9):2606–2610. doi: 10.1073/pnas.81.9.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel W. Electron transport between plastoquinone and chlorophyll a-I in chloroplasts. Biochim Biophys Acta. 1973 Jun 28;305(3):618–631. doi: 10.1016/0005-2728(73)90081-9. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Pröpper A., Krause H. Evidence for complexed plastocyanin as the immediate electron donor of P-700. Biochim Biophys Acta. 1980 Dec 3;593(2):384–399. doi: 10.1016/0005-2728(80)90075-4. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Ratajczak R., Robenek H. Lateral distribution and diffusion of plastocyanin in chloroplast thylakoids. J Cell Biol. 1989 Apr;108(4):1397–1405. doi: 10.1083/jcb.108.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel W. The reduction kinetics of chlorophyll aI as an indicator for proton uptake between the light reactions in chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):506–521. doi: 10.1016/0005-2728(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Trebst A. Localization of electron transport inhibition in plastoquinone reactions. J Bioenerg Biomembr. 1982 Jun;14(3):181–190. doi: 10.1007/BF00745019. [DOI] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Malkin S. Kinetic studies on electron-transport components in isolated chloroplasts. I. The effect of the pool of electron carriers between the two photosystems on P700 changes. Biochim Biophys Acta. 1968 Oct 1;162(3):392–401. doi: 10.1016/0005-2728(68)90125-4. [DOI] [PubMed] [Google Scholar]
- Miller K. R. A chloroplast membrane lacking photosystem I. Changes in unstacked membrane regions. Biochim Biophys Acta. 1980 Aug 5;592(1):143–152. doi: 10.1016/0005-2728(80)90121-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976 Oct 21;62(2):327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., DeWit M. Correlation of structure and function of chloroplast membranes at the supramolecular level. J Cell Biochem. 1984;24(3):261–269. doi: 10.1002/jcb.240240307. [DOI] [PubMed] [Google Scholar]
- Stiehl H. H., Witt H. T. Quantitative treatment of the function of plastoquinone in phostosynthesis. Z Naturforsch B. 1969 Dec;24(12):1588–1598. doi: 10.1515/znb-1969-1219. [DOI] [PubMed] [Google Scholar]
- Venturoli G., Fernández-Velasco J. G., Crofts A. R., Melandri B. A. Demonstration of a collisional interaction of ubiquinol with the ubiquinol-cytochrome c2 oxidoreductase complex in chromatophores from Rhodobacter sphaeroides. Biochim Biophys Acta. 1986 Oct 8;851(3):340–352. doi: 10.1016/0005-2728(86)90070-8. [DOI] [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]


