Abstract
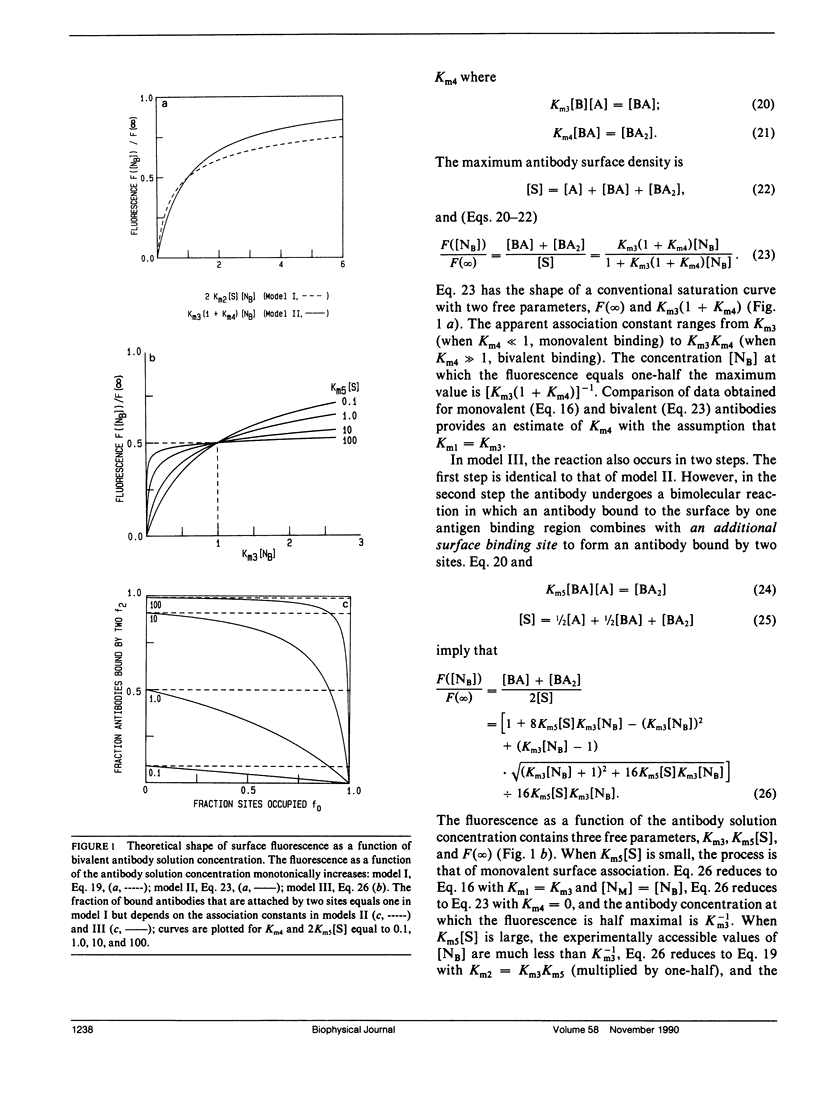
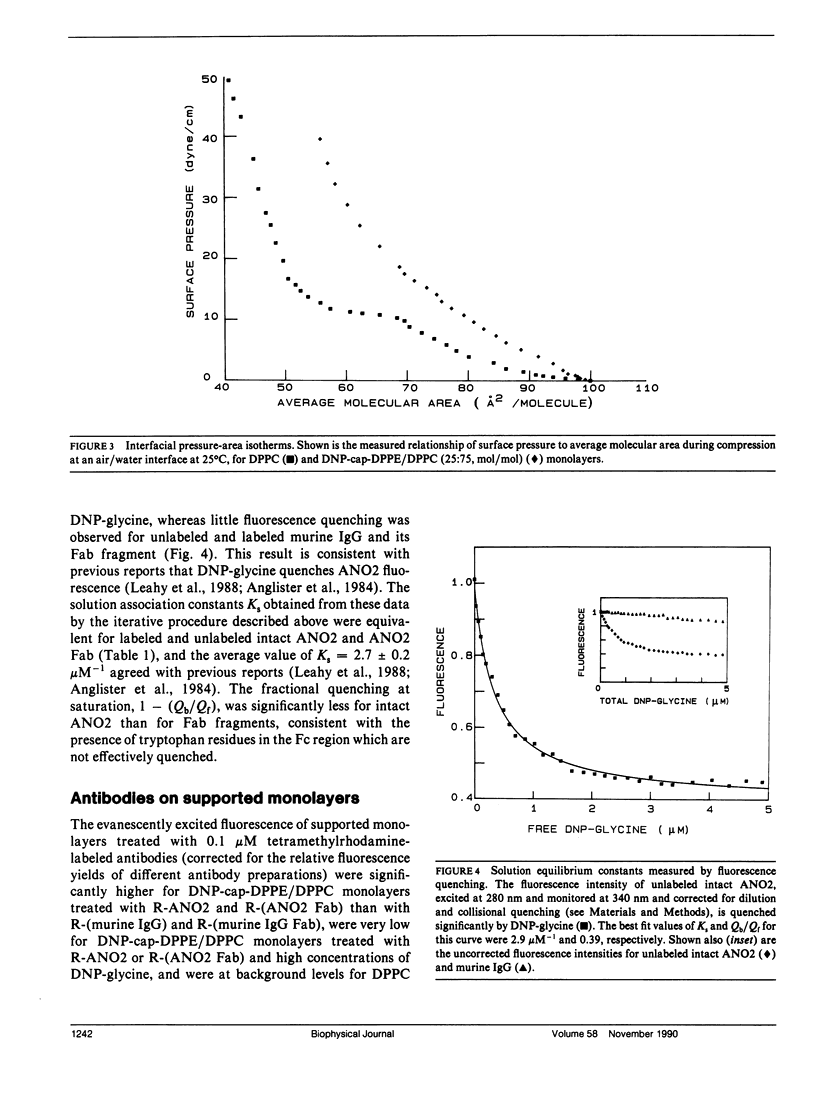
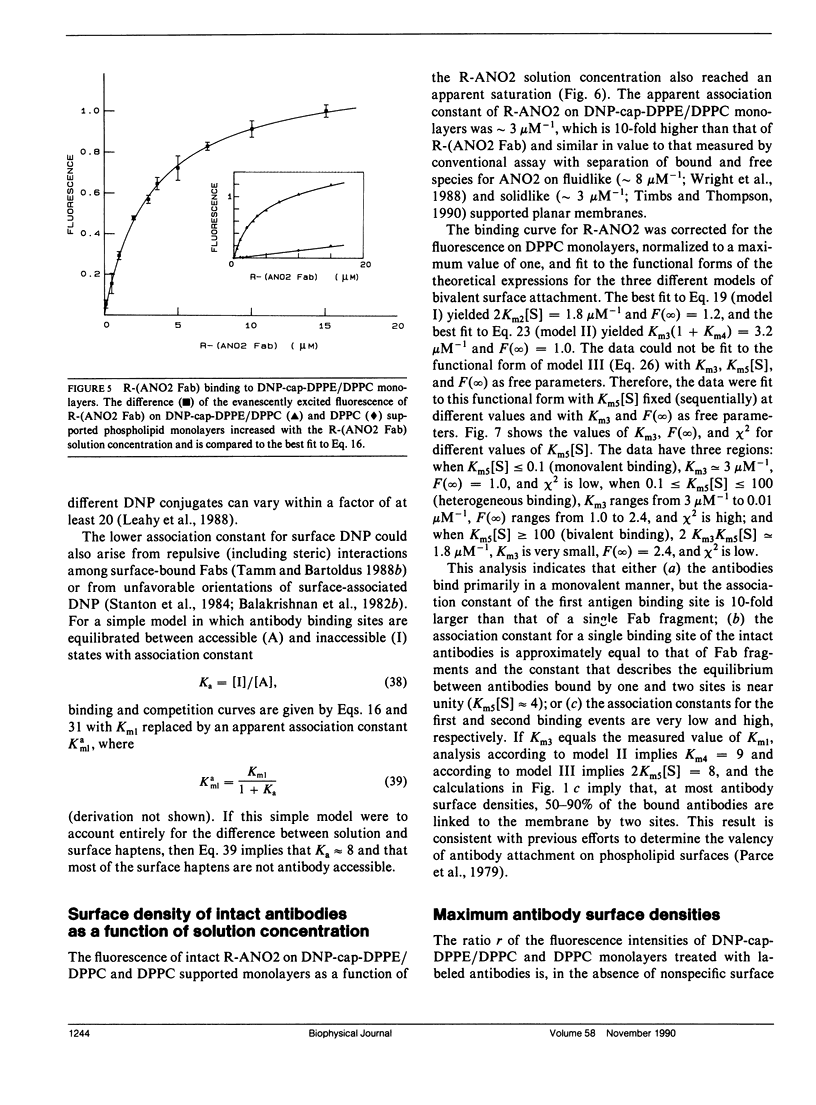
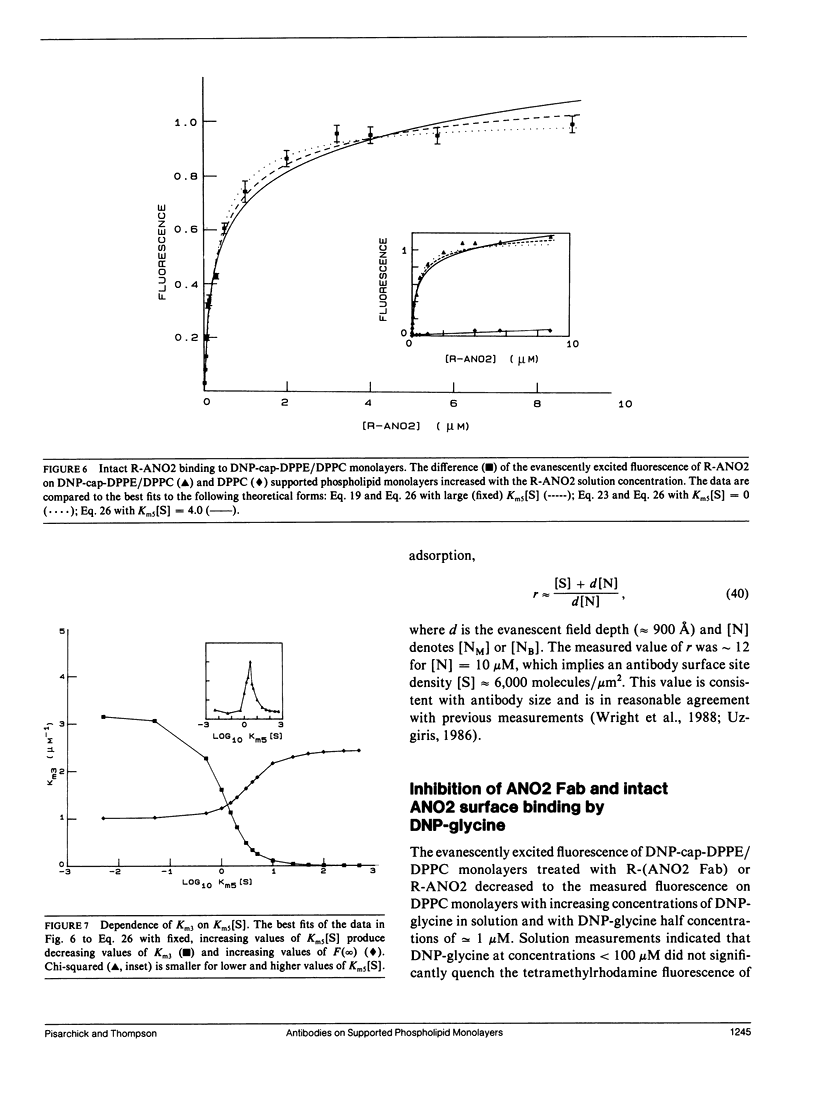
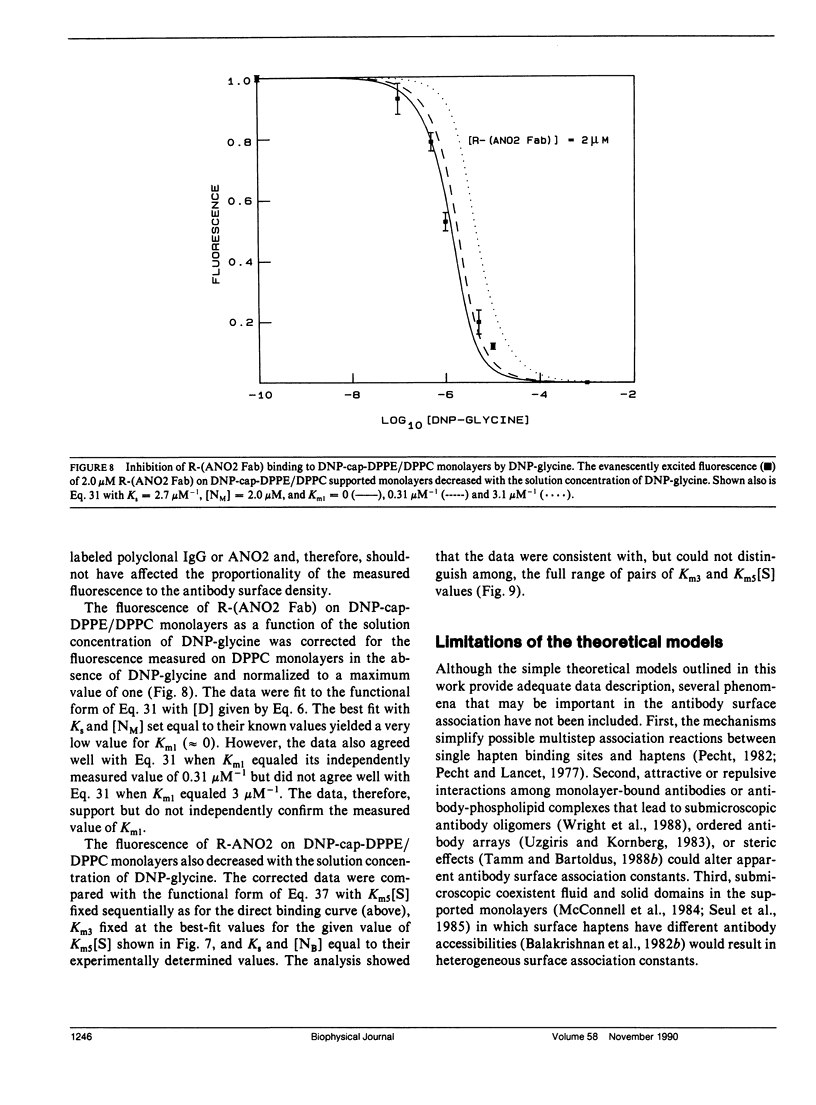
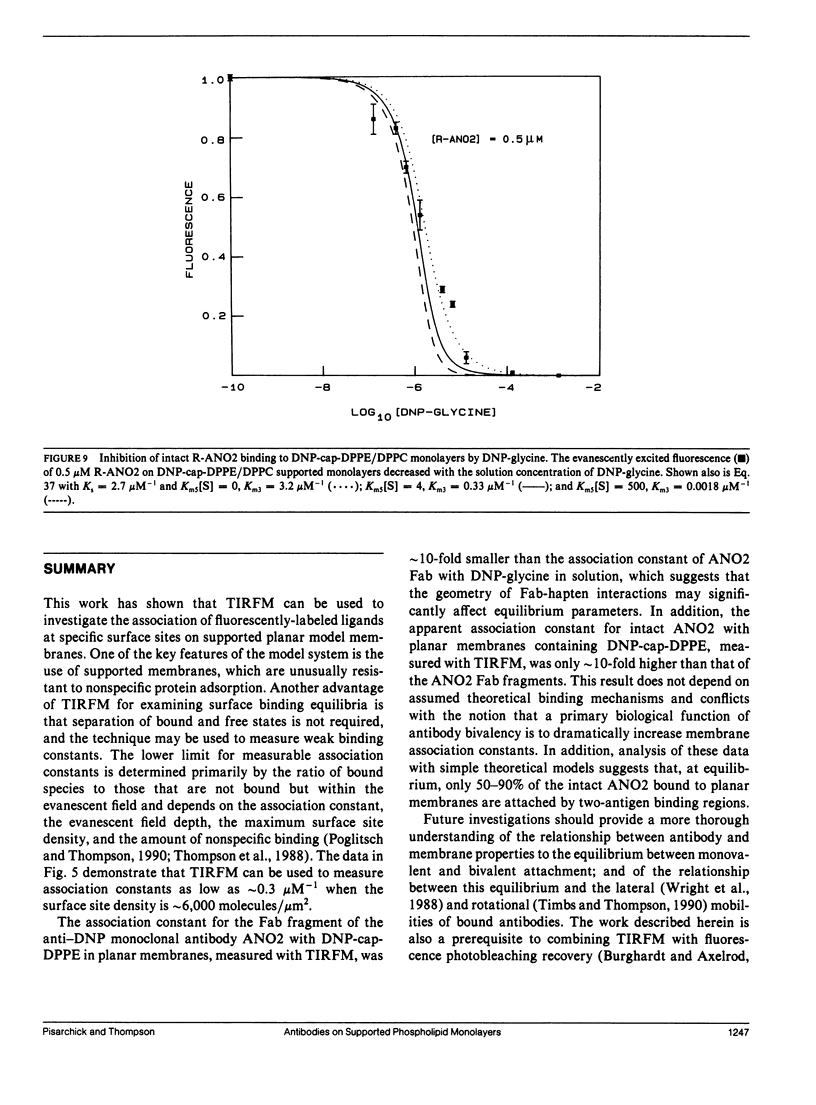
The association of an anti-dinitrophenyl monoclonal antibody and its Fab fragment with supported phospholipid monolayers composed of a mixture of dipalmitoylphosphatidylcholine and dinitrophenyl-conjugated dipalmitoylphosphatidylethanolamine has been characterized with total internal reflection fluorescence microscopy. The surface densities of bound antibodies were measured as a function of the antibody and Fab solution concentrations, and as a function of the solution concentration of dinitrophenylglycine. The apparent association constant of Fab fragments with surface-associated haptens was approximately 10-fold lower than the association constant for haptens in solution, and the apparent surface association constant for intact antibodies was only approximately 10-fold higher than the constant for Fab fragments. Data analysis with simple theoretical models indicated that, at most antibody surface densities, 50-90% of membrane-associated intact antibodies were attached to the surface by two antigen binding sites.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Burghardt T. P., Thompson N. L. Total internal reflection fluorescence. Annu Rev Biophys Bioeng. 1984;13:247–268. doi: 10.1146/annurev.bb.13.060184.001335. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K., Hsu F. J., Hafeman D. G., McConnell H. M. Monoclonal antibodies to a nitroxide lipid hapten. Biochim Biophys Acta. 1982 Sep 13;721(1):30–38. doi: 10.1016/0167-4889(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K., Mehdi S. Q., McConnell H. M. Availability of dinitrophenylated lipid haptens for specific antibody binding depends on the physical properties of host bilayer membranes. J Biol Chem. 1982 Jun 10;257(11):6434–6439. [PubMed] [Google Scholar]
- Burghardt T. P., Axelrod D. Total internal reflection/fluorescence photobleaching recovery study of serum albumin adsorption dynamics. Biophys J. 1981 Mar;33(3):455–467. doi: 10.1016/S0006-3495(81)84906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst S. A., Robertson C. R., Berzofsky J. A. Adsorption of the protein antigen myoglobin affects the binding of conformation-specific monoclonal antibodies. Biophys J. 1988 Apr;53(4):533–539. doi: 10.1016/S0006-3495(88)83133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Goldstein B. A thermodynamic model of binding of flexible bivalent haptens to antibody. Immunochemistry. 1978 May;15(5):307–313. doi: 10.1016/0161-5890(78)90091-3. [DOI] [PubMed] [Google Scholar]
- Dower S. K., DeLisi C., Titus J. A., Segal D. M. Mechanism of binding of multivalent immune complexes to Fc receptors. 1. Equilibrium binding. Biochemistry. 1981 Oct 27;20(22):6326–6334. doi: 10.1021/bi00525a007. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Erickson J., Goldstein B., Holowka D., Baird B. The effect of receptor density on the forward rate constant for binding of ligands to cell surface receptors. Biophys J. 1987 Oct;52(4):657–662. doi: 10.1016/S0006-3495(87)83258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J., Kane P., Goldstein B., Holowka D., Baird B. Cross-linking of IgE-receptor complexes at the cell surface: a fluorescence method for studying the binding of monovalent and bivalent haptens to IgE. Mol Immunol. 1986 Jul;23(7):769–781. doi: 10.1016/0161-5890(86)90089-1. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Posner R. G., Torney D. C., Erickson J., Holowka D., Baird B. Competition between solution and cell surface receptors for ligand. Dissociation of hapten bound to surface antibody in the presence of solution antibody. Biophys J. 1989 Nov;56(5):955–966. doi: 10.1016/S0006-3495(89)82741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., von Tscharner V., McConnell H. M. Specific antibody-dependent interactions between macrophages and lipid haptens in planar lipid monolayers. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4552–4556. doi: 10.1073/pnas.78.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J. N., Kranz D. M., Jameson D. M., Voss E. W., Jr Thermodynamic properties of ligand binding by monoclonal anti-fluorescyl antibodies. Biochemistry. 1986 Aug 12;25(16):4602–4609. doi: 10.1021/bi00364a022. [DOI] [PubMed] [Google Scholar]
- Lancet D., Pecht I. Spectroscopic and immunochemical studies with nitrobenzoxadiazolealanine, a fluorescent dinitrophenyl analogue. Biochemistry. 1977 Nov 15;16(23):5150–5157. doi: 10.1021/bi00642a031. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Rule G. S., Whittaker M. M., McConnell H. M. Sequences of 12 monoclonal anti-dinitrophenyl spin-label antibodies for NMR studies. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3661–3665. doi: 10.1073/pnas.85.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- McGuigan J. E., Eisen H. N. Differences in spectral properties and tryptophan content among rabbit anti-2,4-dinitrophenyl antibodies of the gamma G-immunologbulin class. Biochemistry. 1968 May;7(5):1919–1928. doi: 10.1021/bi00845a041. [DOI] [PubMed] [Google Scholar]
- Nygren H., Werthen M., Stenberg M. Kinetics of antibody binding to solid-phase-immobilised antigen. Effect of diffusion rate limitation and steric interaction. J Immunol Methods. 1987 Jul 16;101(1):63–71. doi: 10.1016/0022-1759(87)90217-1. [DOI] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. High-order fluorescence fluctuation analysis of model protein clusters. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6148–6152. doi: 10.1073/pnas.86.16.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrossian A., Owicki J. C. Interaction of antibodies with liposomes bearing fluorescent haptens. Biochim Biophys Acta. 1984 Oct 3;776(2):217–227. doi: 10.1016/0005-2736(84)90211-6. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Poglitsch C. L., Thompson N. L. Interaction of antibodies with Fc receptors in substrate-supported planar membranes measured by total internal reflection fluorescence microscopy. Biochemistry. 1990 Jan 9;29(1):248–254. doi: 10.1021/bi00453a033. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton S. G., Kantor A. B., Petrossian A., Owicki J. C. Location and dynamics of a membrane-bound fluorescent hapten. A spectroscopic study. Biochim Biophys Acta. 1984 Oct 3;776(2):228–236. doi: 10.1016/0005-2736(84)90212-8. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Seul M., McConnell H. M. Lateral diffusion of specific antibodies bound to lipid monolayers on alkylated substrates. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1169–1173. doi: 10.1073/pnas.83.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui S. F., Urumow T., Sackmann E. Interaction of insulin receptors with lipid bilayers and specific and nonspecific binding of insulin to supported membranes. Biochemistry. 1988 Sep 20;27(19):7463–7469. doi: 10.1021/bi00419a044. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Lateral diffusion and fluorescence microscope studies on a monoclonal antibody specifically bound to supported phospholipid bilayers. Biochemistry. 1988 Mar 8;27(5):1450–1457. doi: 10.1021/bi00405a009. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Axelrod D. Immunoglobulin surface-binding kinetics studied by total internal reflection with fluorescence correlation spectroscopy. Biophys J. 1983 Jul;43(1):103–114. doi: 10.1016/S0006-3495(83)84328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbs M. M., Thompson N. L. Slow rotational mobilities of antibodies and lipids associated with substrate-supported phospholipid monolayers as measured by polarized fluorescence photobleaching recovery. Biophys J. 1990 Aug;58(2):413–428. doi: 10.1016/S0006-3495(90)82387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E. Supported phospholipid bilayers for two-dimensional protein crystallization. Biochem Biophys Res Commun. 1986 Jan 29;134(2):819–826. doi: 10.1016/s0006-291x(86)80494-6. [DOI] [PubMed] [Google Scholar]
- Wright L. L., Palmer A. G., 3rd, Thompson N. L. Inhomogeneous translational diffusion of monoclonal antibodies on phospholipid Langmuir-Blodgett films. Biophys J. 1988 Sep;54(3):463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys J. 1981 Nov;36(2):421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


