Abstract
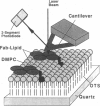
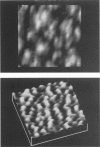
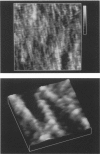
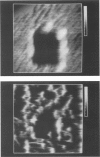
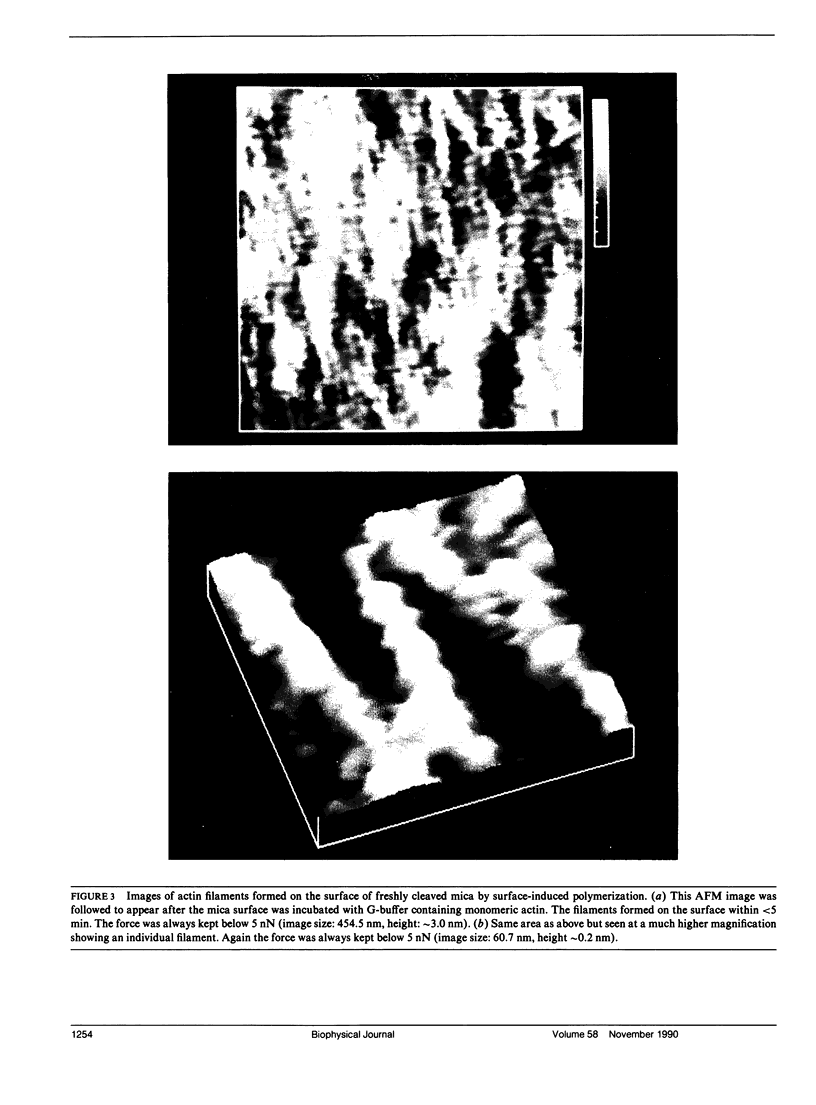
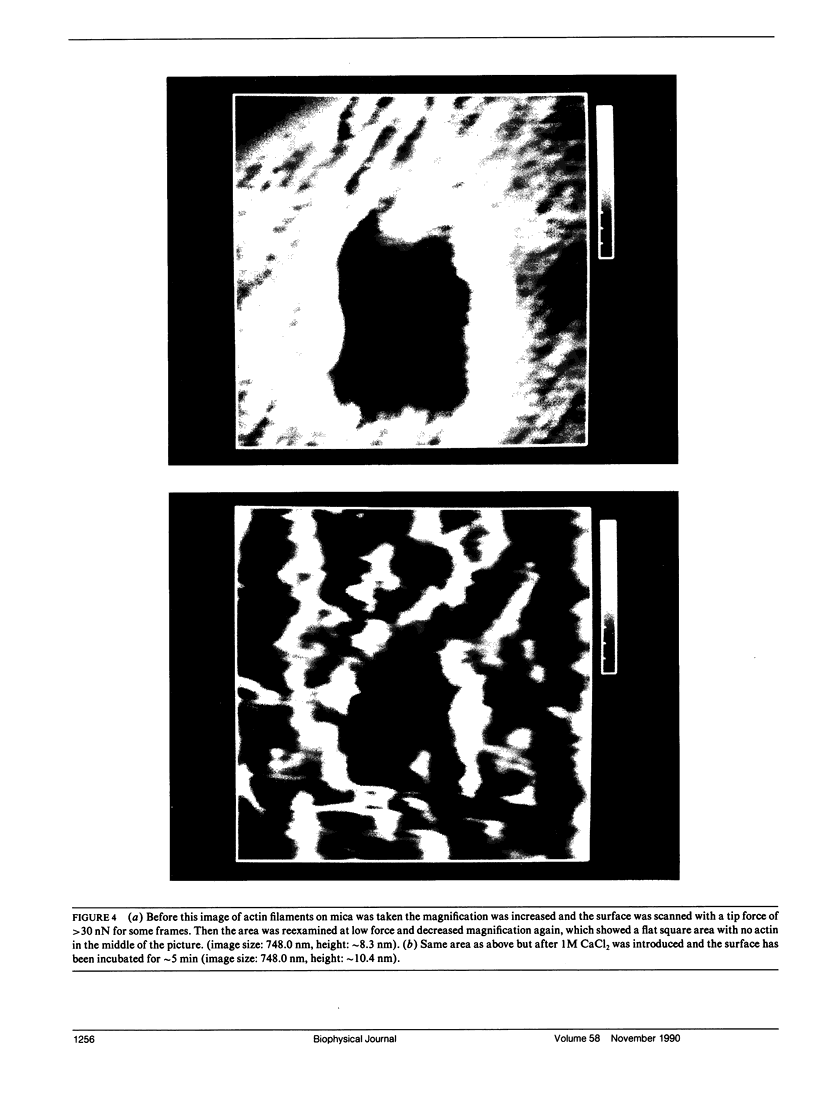
Samples of supported planar lipid-protein membranes and actin filaments on mica were imaged by atomic force microscopy (AFM). The samples were fully submerged in buffer at room temperature during imaging. Individual proteins bound to the reconstituted membrane were distinguishable; some structural details could be resolved. Also, surface-induced, self-assembling of actin filaments on mica could be observed. Monomeric subunits were imaged on individual actin filaments. The filaments could be manipulated on or removed from the surface by the tip of the AFM. The process of the decoupling of the filamentous network from the surface upon changing the ionic conditions was imaged in real time.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balakrishnan K., Hsu F. J., Hafeman D. G., McConnell H. M. Monoclonal antibodies to a nitroxide lipid hapten. Biochim Biophys Acta. 1982 Sep 13;721(1):30–38. doi: 10.1016/0167-4889(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1975;44:639–667. doi: 10.1146/annurev.bi.44.070175.003231. [DOI] [PubMed] [Google Scholar]
- Drake B., Prater C. B., Weisenhorn A. L., Gould S. A., Albrecht T. R., Quate C. F., Cannell D. S., Hansma H. G., Hansma P. K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989 Mar 24;243(4898):1586–1589. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- Egger M., Heyn S. P., Gaub H. E. Two-dimensional recognition pattern of lipid-anchored Fab' fragments. Biophys J. 1990 Mar;57(3):669–673. doi: 10.1016/S0006-3495(90)82586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma P. K., Elings V. B., Marti O., Bracker C. E. Scanning tunneling microscopy and atomic force microscopy: application to biology and technology. Science. 1988 Oct 14;242(4876):209–216. doi: 10.1126/science.3051380. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Laliberte A., Gicquaud C. Polymerization of actin by positively charged liposomes. J Cell Biol. 1988 Apr;106(4):1221–1227. doi: 10.1083/jcb.106.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Biochem Biophys Res Commun. 1980 Sep 16;96(1):18–27. doi: 10.1016/0006-291x(80)91175-4. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Zingsheim H. P. Apparent holes in rotary shadowed proteins: dependence on angle of shadowing and replica thickness. J Microsc. 1979 Nov;117(2):313–315. doi: 10.1111/j.1365-2818.1979.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Watts T. H., Gaub H. E., McConnell H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy transfer in an evanescent wave-field. Nature. 1986 Mar 13;320(6058):179–181. doi: 10.1038/320179a0. [DOI] [PubMed] [Google Scholar]