Abstract
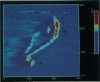
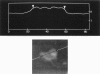
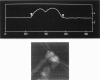
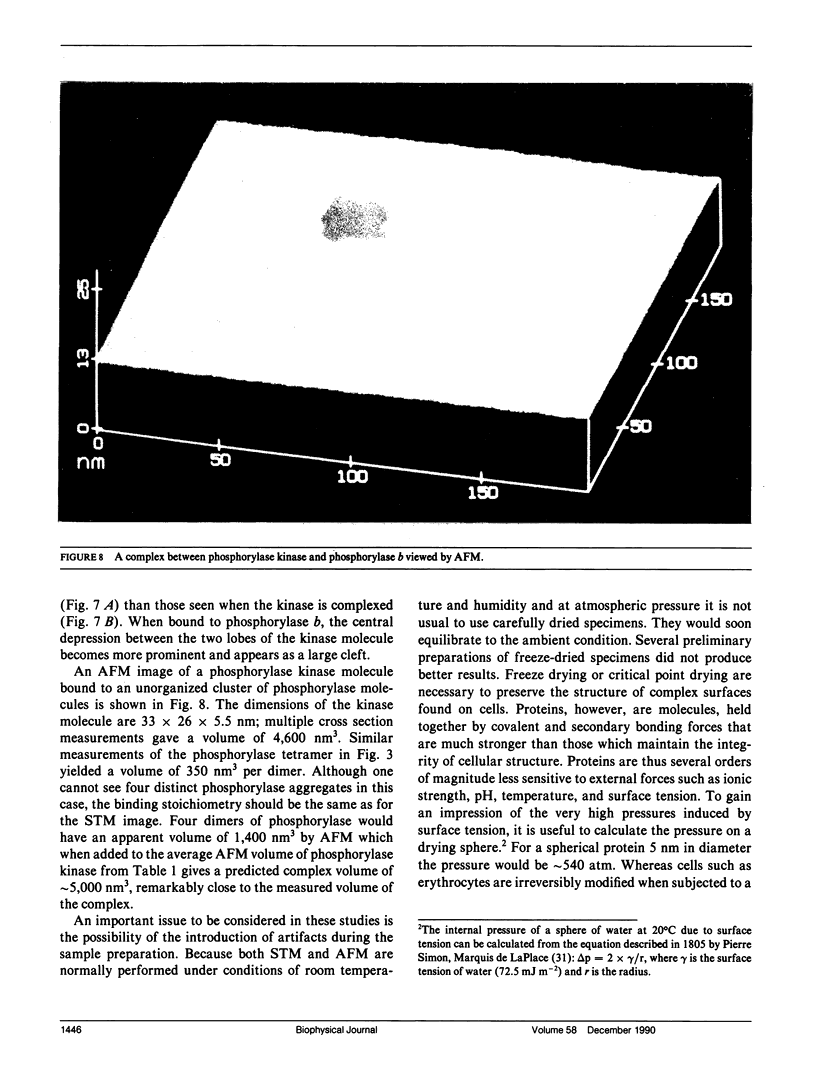
In skeletal muscle the activation of phosphorylase b is catalyzed by phosphorylase kinase. Both enzymes occur in vivo as part of a multienzyme complex. The two enzymes have been imaged by atomic force microscopy and the results compared to those previously found by scanning tunneling microscopy. Scanning tunneling microscopy and atomic force microscopy have been used to view complexes between the activating enzyme phosphorylase kinase and its substrate phosphorylase b. Changes in the size and shape of phosphorylase kinase were observed when it bound phosphorylase b.
Full text
PDF
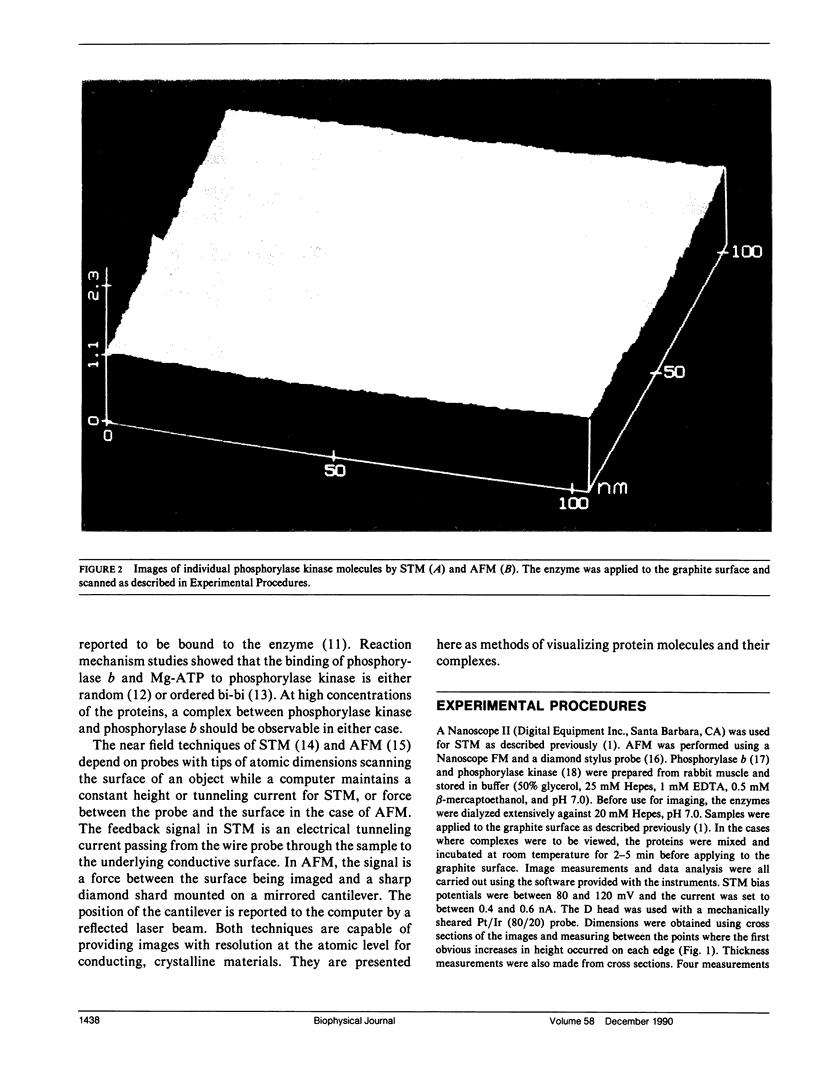
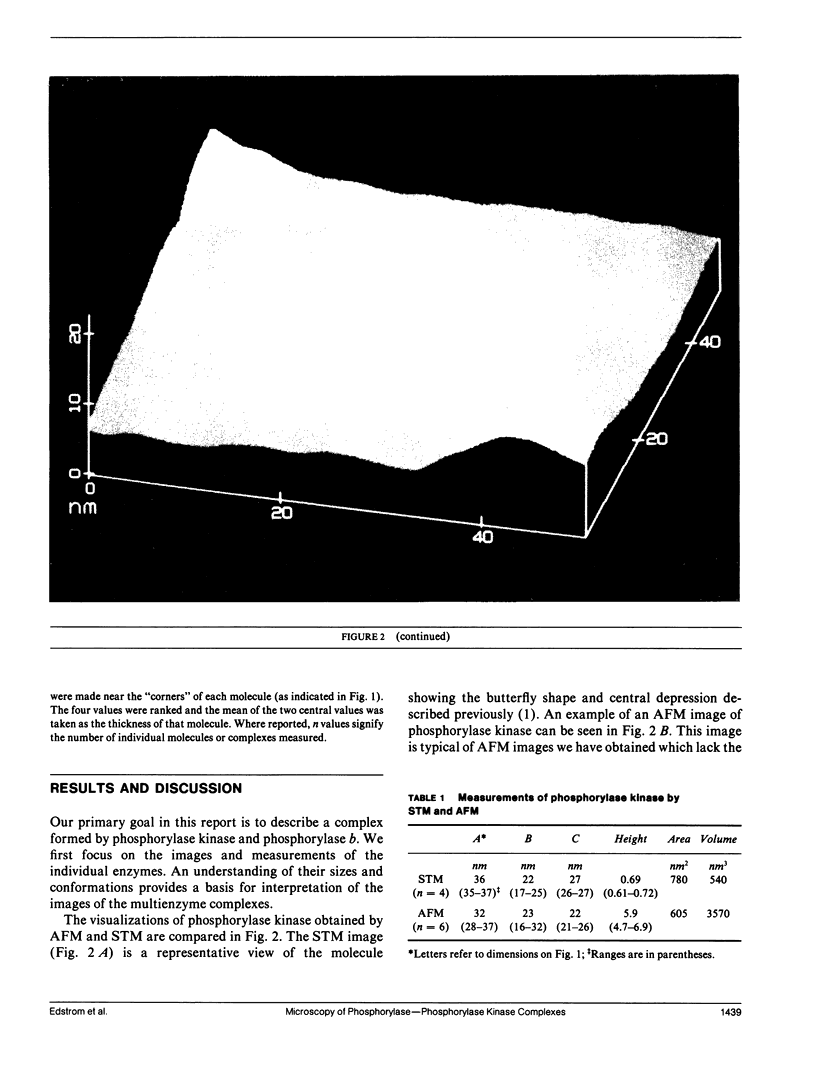
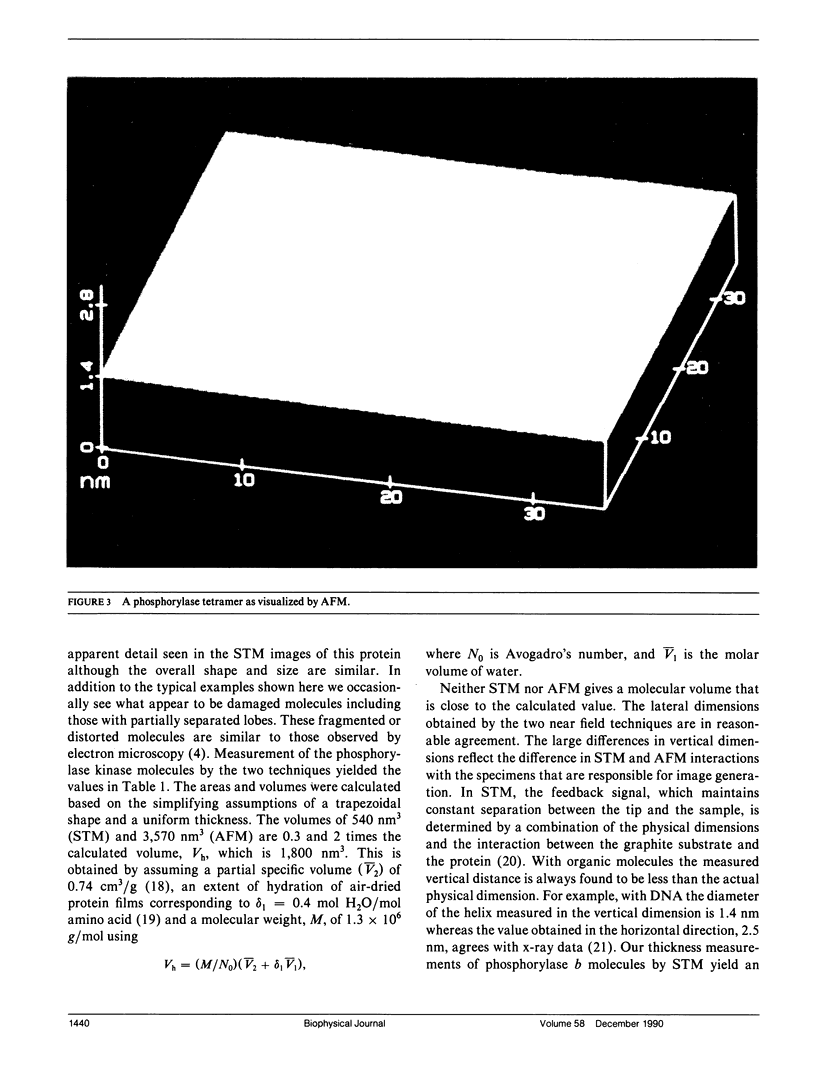
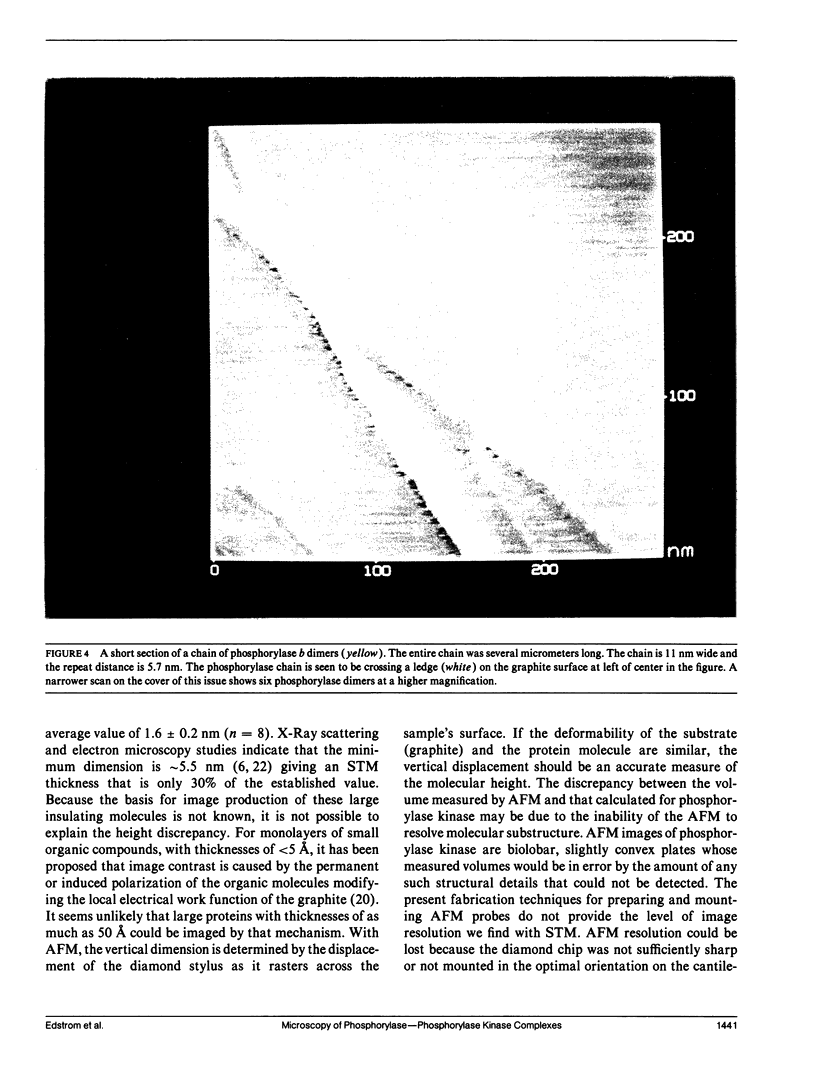
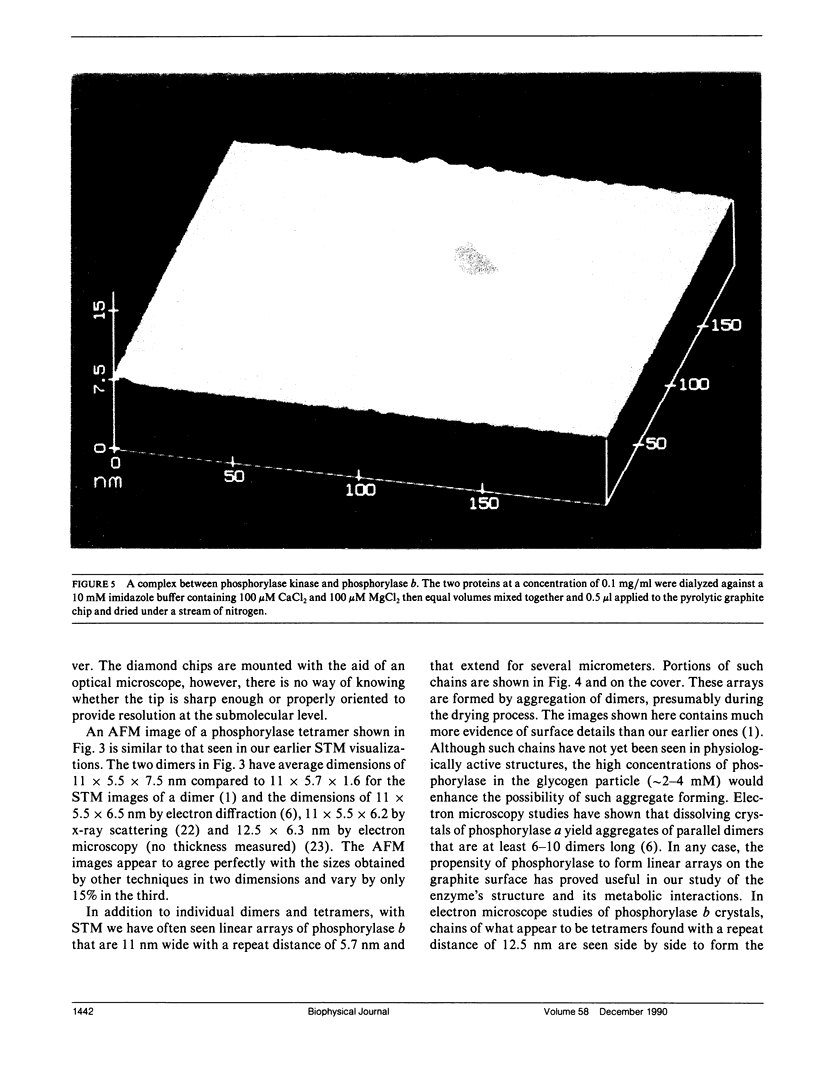
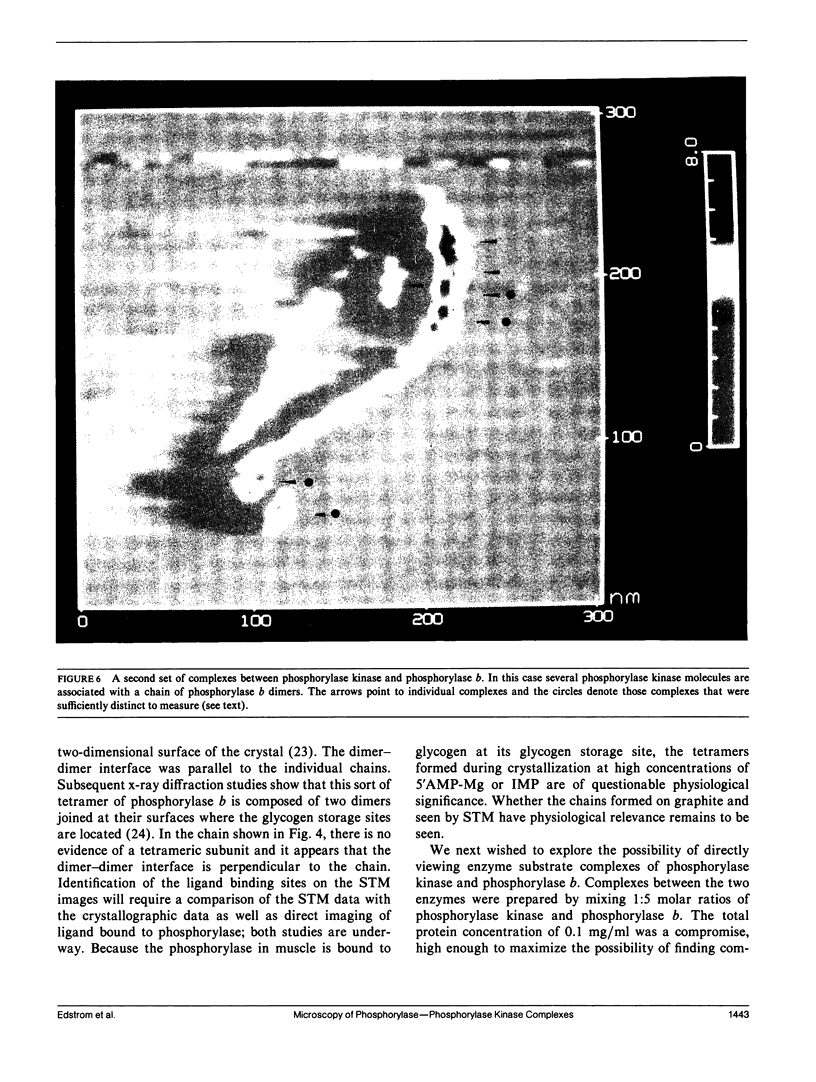
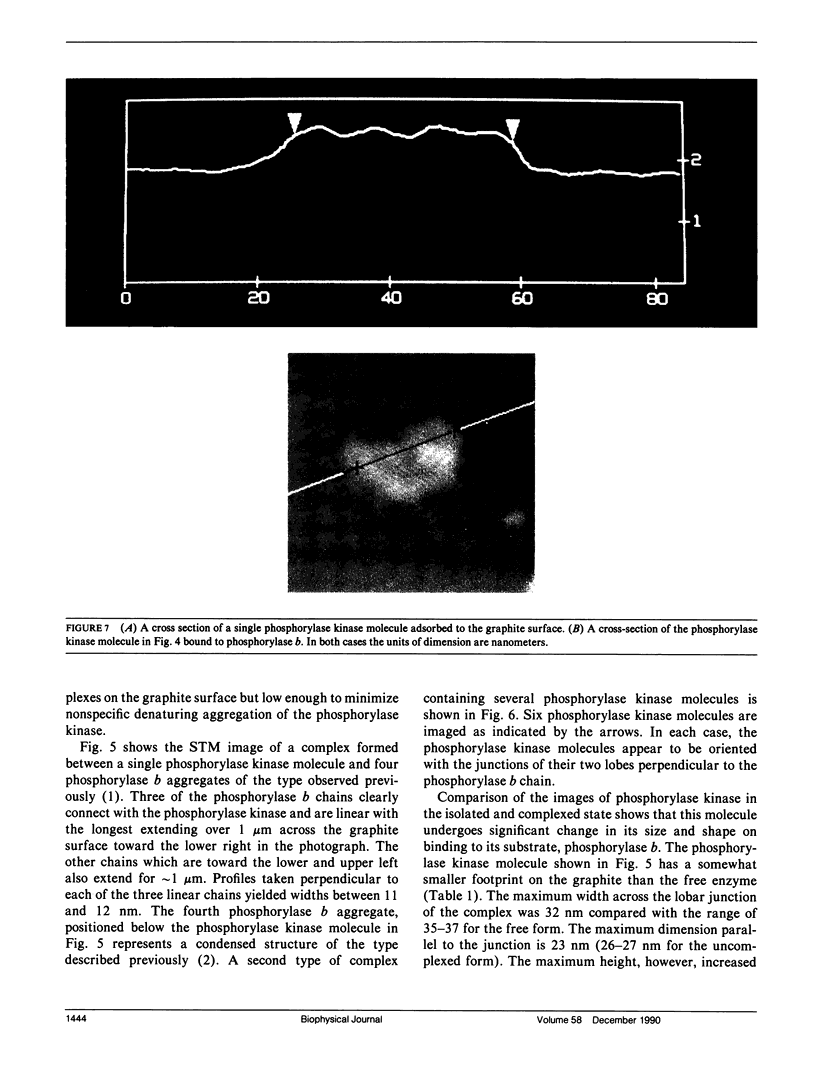
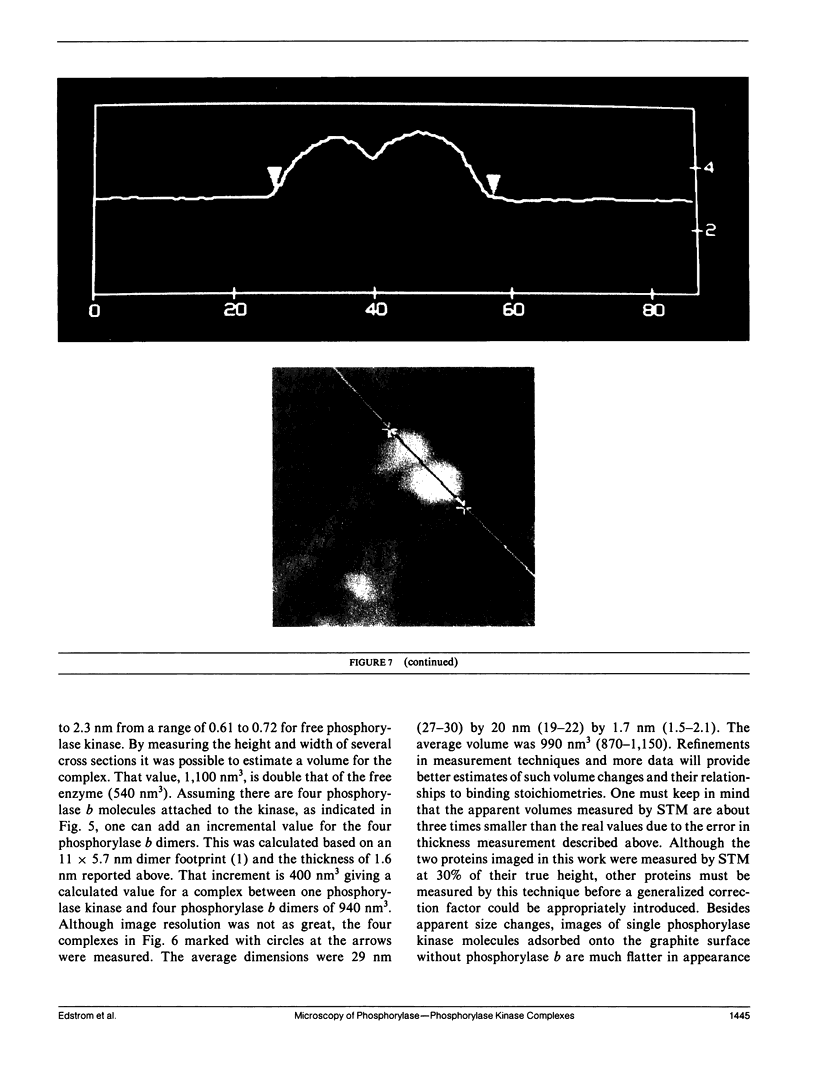


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barford D., Johnson L. N. The allosteric transition of glycogen phosphorylase. Nature. 1989 Aug 24;340(6235):609–616. doi: 10.1038/340609a0. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Chignell D. A., Gratzer W. B., Valentine R. C. Subunit interaction in native and modified muscle phosphorylases. Biochemistry. 1968 Mar;7(3):1082–1089. doi: 10.1021/bi00843a028. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of phosphorylase kinase in the nervous and hormonal control of glycogenolysis in muscle. Biochem Soc Symp. 1974;(39):51–73. [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Johnson L. N. Electron microscopy of phosphorylase b crystals. J Mol Biol. 1972 Mar 14;64(3):693–695. doi: 10.1016/0022-2836(72)90092-7. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Meinke M. H., Yang X., Yang R., Evans D. F. Direct observation of phosphorylase kinase and phosphorylase b by scanning tunneling microscopy. Biochemistry. 1989 Jun 13;28(12):4939–4942. doi: 10.1021/bi00438a004. [DOI] [PubMed] [Google Scholar]
- Halle D., Yedgar S. Mild pressure induces resistance of erythrocytes to hemolysis by snake venom phospholipase A2. Biophys J. 1988 Sep;54(3):393–396. doi: 10.1016/S0006-3495(88)82972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J. N., Ely K. R., Edmundson A. B. Pressure-induced conformational changes in a human Bence-Jones protein (Mcg). Biochemistry. 1985 Jul 2;24(14):3453–3459. doi: 10.1021/bi00335a010. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Häner M., Wurtz M. The wrapping phenomenon in air-dried and negatively stained preparations. Ultramicroscopy. 1982;9(1-2):139–150. doi: 10.1016/0304-3991(82)90236-4. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Crystal structure of hen egg-white lysozyme at a hydrostatic pressure of 1000 atmospheres. J Mol Biol. 1987 Jan 5;193(1):157–170. doi: 10.1016/0022-2836(87)90634-6. [DOI] [PubMed] [Google Scholar]
- Lee G., Arscott P. G., Bloomfield V. A., Evans D. F. Scanning tunneling microscopy of nucleic acids. Science. 1989 Apr 28;244(4903):475–477. doi: 10.1126/science.2470146. [DOI] [PubMed] [Google Scholar]
- Mantulin W. W., Pownall H. J. Reversible folding reactions of human apolipoprotein A-I: pressure and guanidinium chloride effects. Biochim Biophys Acta. 1985 Sep 11;836(2):215–221. doi: 10.1016/0005-2760(85)90069-4. [DOI] [PubMed] [Google Scholar]
- Müller K., Seifert T., Jaenicke R. High pressure dissociation of lactate dehydrogenase from Bacillus stearothermophilus and reconstitution of the enzyme after denaturation in 6 M guanidine hydrochloride. Eur Biophys J. 1984;11(2):87–94. doi: 10.1007/BF00276623. [DOI] [PubMed] [Google Scholar]
- Paudel H. K., Carlson G. M. Inhibition of the catalytic subunit of phosphorylase kinase by its alpha/beta subunits. J Biol Chem. 1987 Sep 5;262(25):11912–11915. [PubMed] [Google Scholar]
- Puchwein G., Kratky O., Gölker C. F., Helmreich E. Small-angle x-ray scattering measurements on rabbit muscle glycogen phosphorylase dimer b and tetramer b. Biochemistry. 1970 Nov 24;9(24):4691–4698. doi: 10.1021/bi00826a011. [DOI] [PubMed] [Google Scholar]
- Sprang S. R., Acharya K. R., Goldsmith E. J., Stuart D. I., Varvill K., Fletterick R. J., Madsen N. B., Johnson L. N. Structural changes in glycogen phosphorylase induced by phosphorylation. Nature. 1988 Nov 17;336(6196):215–221. doi: 10.1038/336215a0. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Graves D. J. Kinetic mechanism and specificity of the phosphorylase kinase reaction. J Biol Chem. 1978 Apr 10;253(7):2196–2202. [PubMed] [Google Scholar]
- Trempe M. R., Carlson G. M., Hainfeld J. F., Furcinitti P. S., Wall J. S. Analyses of phosphorylase kinase by transmission and scanning transmission electron microscopy. J Biol Chem. 1986 Feb 25;261(6):2882–2889. [PubMed] [Google Scholar]