Abstract
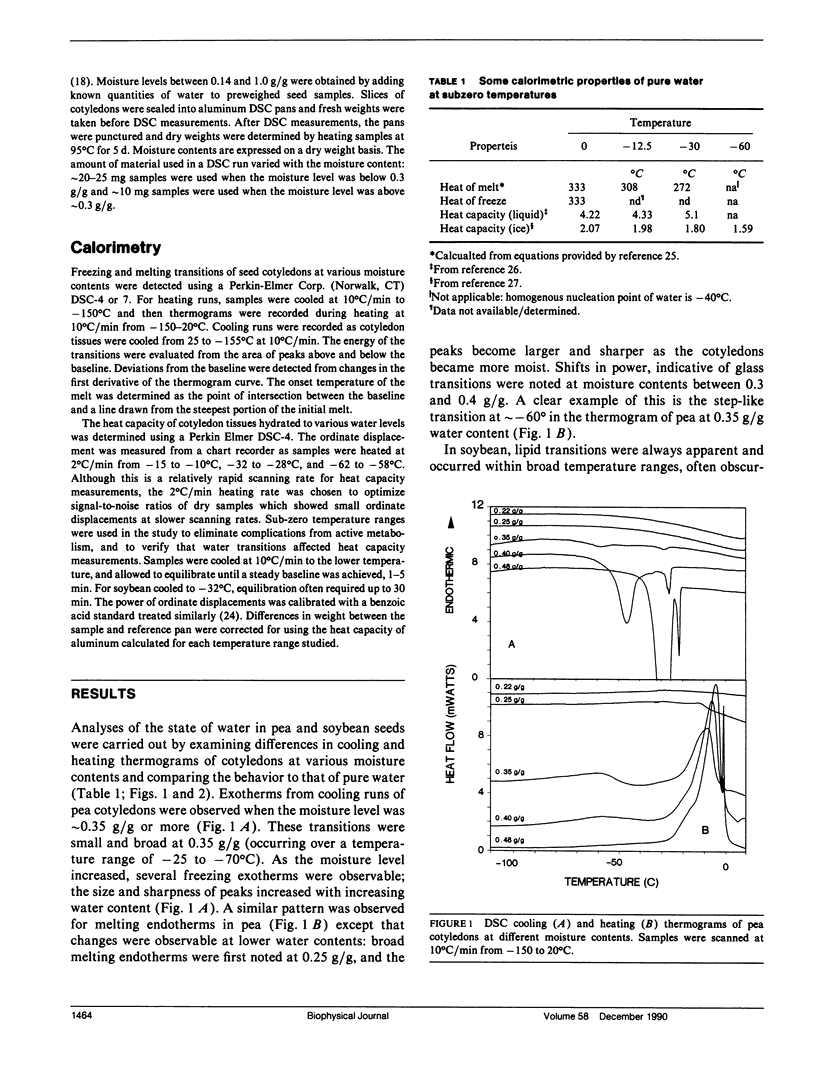
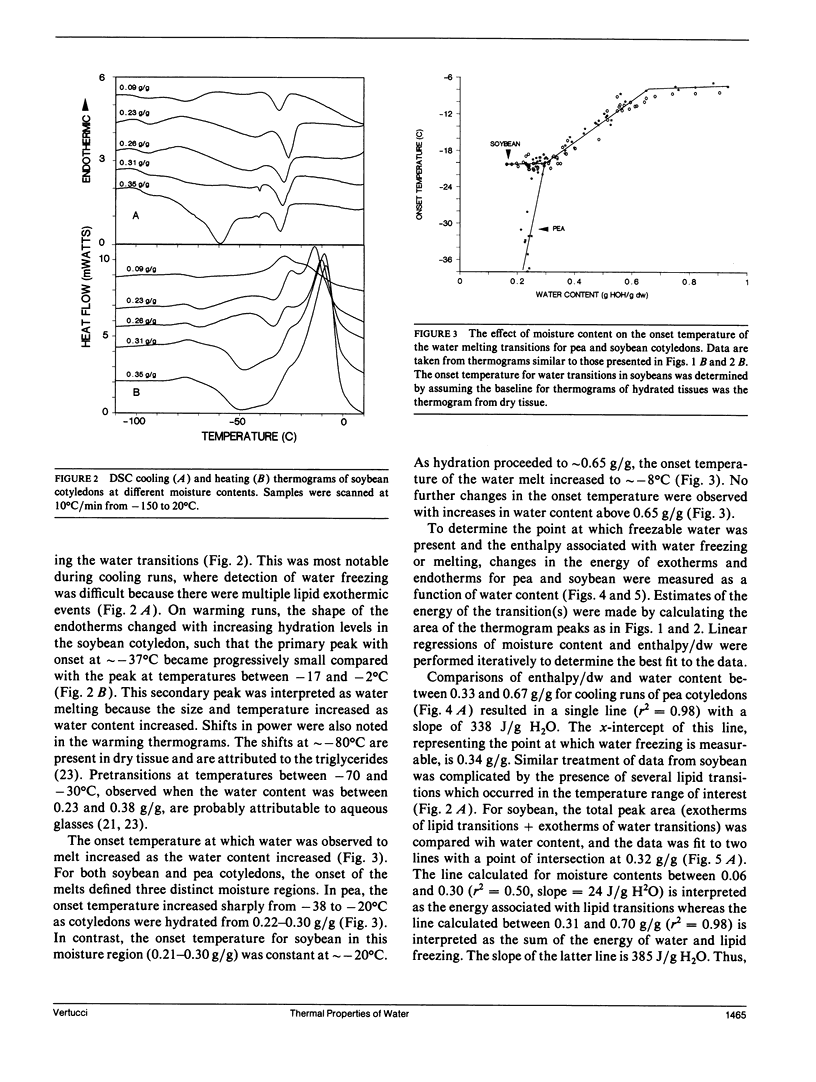
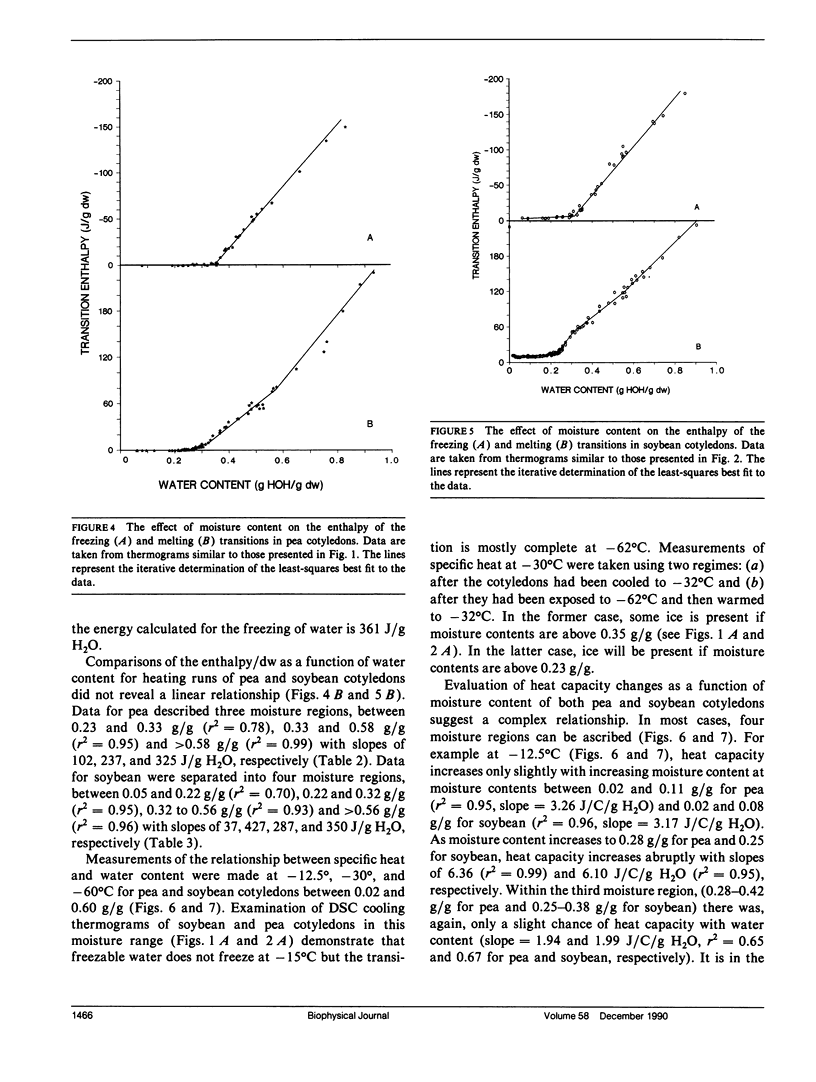
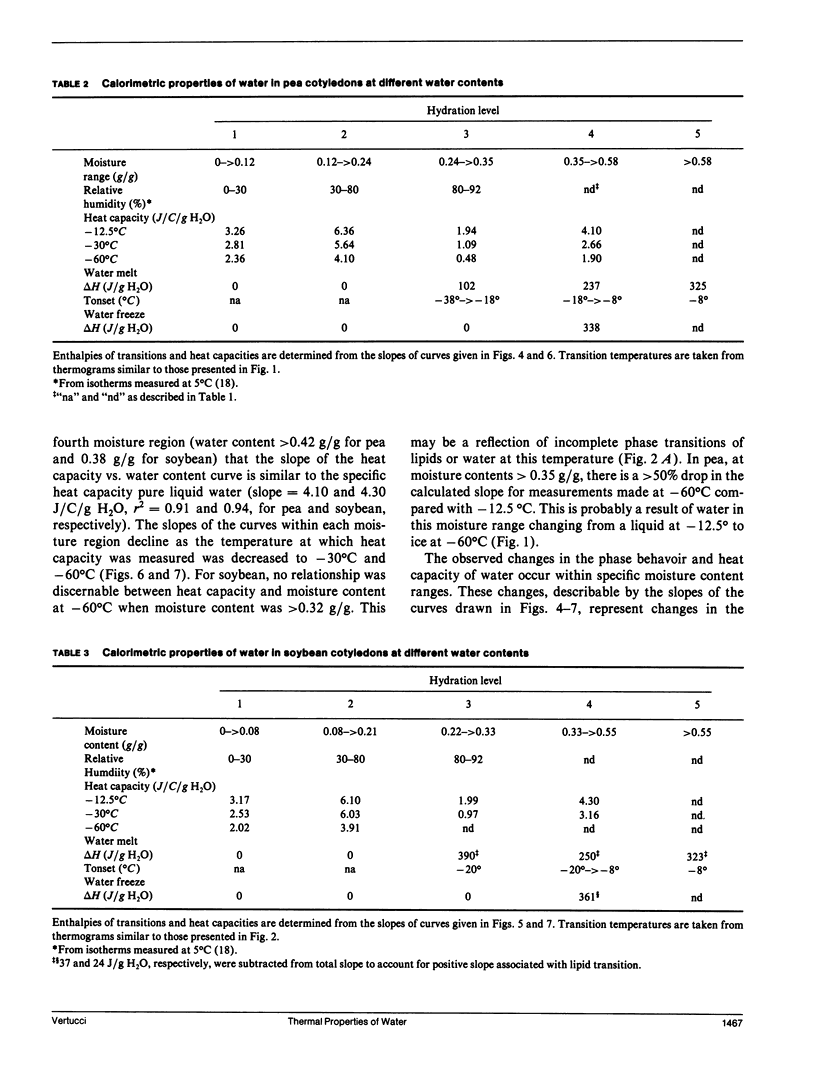
To understand the physical state of water in hydrating biological tissues, thermodynamic properties of water in cotyledons of pea and soybean with moisture contents ranging from 0.01 g H2O/g dw to 1.0 g H2O/g dw were studied using differential scanning calorimetry. The heat capacity of the tissues increased abruptly at moisture contents above 0.08 and 0.12 g H2O/g dw for soybean and pea cotyledons, respectively. Melting transitions of water were observed at moisture contents >0.23 and 0.26 g H2O/g dw for soybean and pea. However, freezing of water was not observed unless moisture contents exceeded 0.33-0.35 g H2O/g dw. In both seed tissues, the temperatures of the freezing and melting varied with moisture content and showed hysterisis. The energy of the transition also varied with moisture content and was similar to the heats of fusion and crystallization of pure water only at moisture contents >0.54 and 0.58 gH2O/g dw for soybean and pea seeds, respectively. The thermal properties of water change distinctly as seed moisture content changes: at least five states or water can be identified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni F., Careri G., Clegg J. S. Dielectric properties of Artemia cysts at low water contents. Evidence for a percolative transition. Biophys J. 1989 Feb;55(2):331–338. doi: 10.1016/S0006-3495(89)82809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni F, Careri G, Leopold AC. Critical exponents of protonic percolation in maize seeds. Phys Rev A Gen Phys. 1989 Sep 1;40(5):2803–2805. doi: 10.1103/physreva.40.2803. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Hunter K. E., Fullerton G. D. Quench cooled ice crystal imprint size: a micro-method for study of macromolecular hydration. Scanning Microsc. 1988 Jun;2(2):885–898. [PubMed] [Google Scholar]
- Cameron I. L., Ord V. A., Fullerton G. D. Water of hydration in the intra- and extra-cellular environment of human erythrocytes. Biochem Cell Biol. 1988 Nov;66(11):1186–1199. doi: 10.1139/o88-136. [DOI] [PubMed] [Google Scholar]
- Careri G., Giansanti A., Gratton E. Lysozyme film hydration events: an ir and gravimetric study. Biopolymers. 1979 May;18(5):1187–1203. doi: 10.1002/bip.1979.360180512. [DOI] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks F., Eagland D. The role of solvent interactions in protein conformation. CRC Crit Rev Biochem. 1975 Aug;3(2):165–219. doi: 10.3109/10409237509102556. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Rüegg M., Moor U., Blanc B. Hydration and thermal denaturation of beta-lactoglobulin. A calorimetric study. Biochim Biophys Acta. 1975 Aug 19;400(2):334–342. doi: 10.1016/0005-2795(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Simatos D., Faure M., Bonjour E., Couach M. The physical state of water at low temperatures in plasma with different water contents as studied by differential thermal analysis and differential scanning calorimetry. Cryobiology. 1975 Jun;12(3):202–208. doi: 10.1016/0011-2240(75)90018-8. [DOI] [PubMed] [Google Scholar]
- Vertucci C. W. Effects of cooling rate on seeds exposed to liquid nitrogen temperatures. Plant Physiol. 1989 Aug;90(4):1478–1485. doi: 10.1104/pp.90.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. The relationship between water binding and desiccation tolerance in tissues. Plant Physiol. 1987;85:232–238. doi: 10.1104/pp.85.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. Water binding in legume seeds. Plant Physiol. 1987;85:224–231. doi: 10.1104/pp.85.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W. Relationship between Thermal Transitions and Freezing Injury in Pea and Soybean Seeds. Plant Physiol. 1989 Jul;90(3):1121–1128. doi: 10.1104/pp.90.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Leopold A. C. The glassy state in corn embryos. Plant Physiol. 1989 Mar;89(3):977–981. doi: 10.1104/pp.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. H., Rupley J. A. Protein--water interactions. Heat capacity of the lysozyme--water system. Biochemistry. 1979 Jun 12;18(12):2654–2661. doi: 10.1021/bi00579a035. [DOI] [PubMed] [Google Scholar]


