Abstract
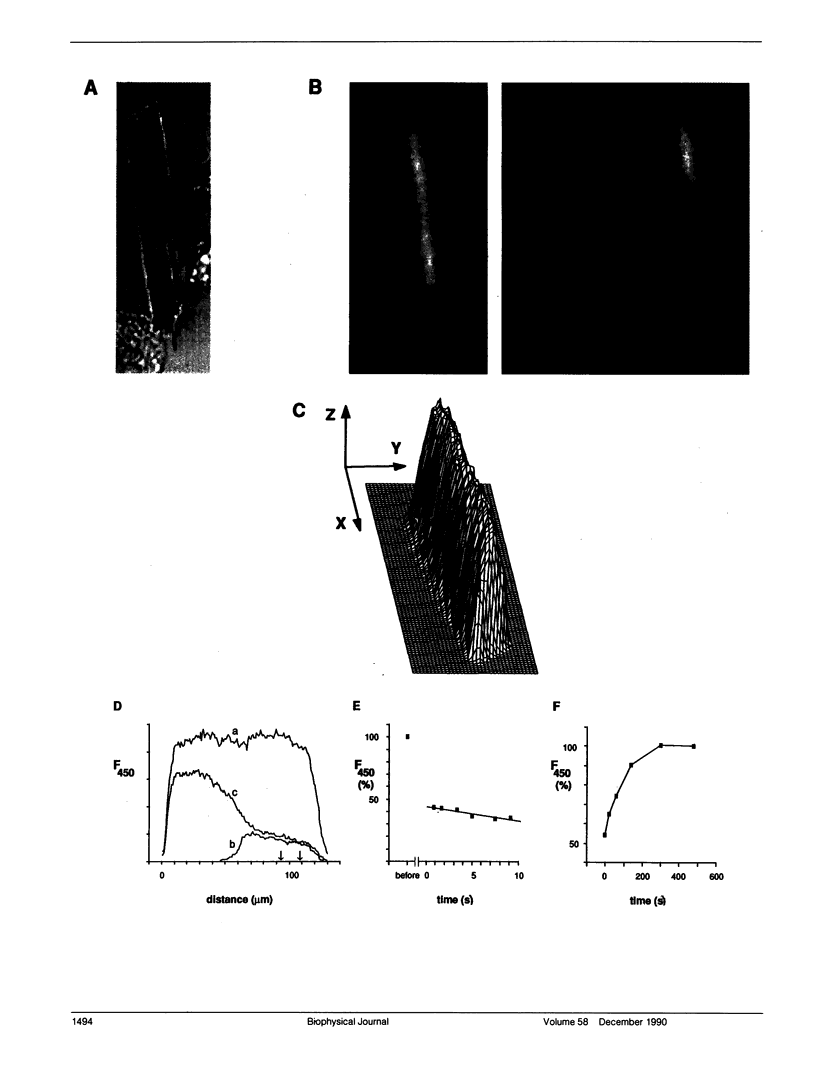
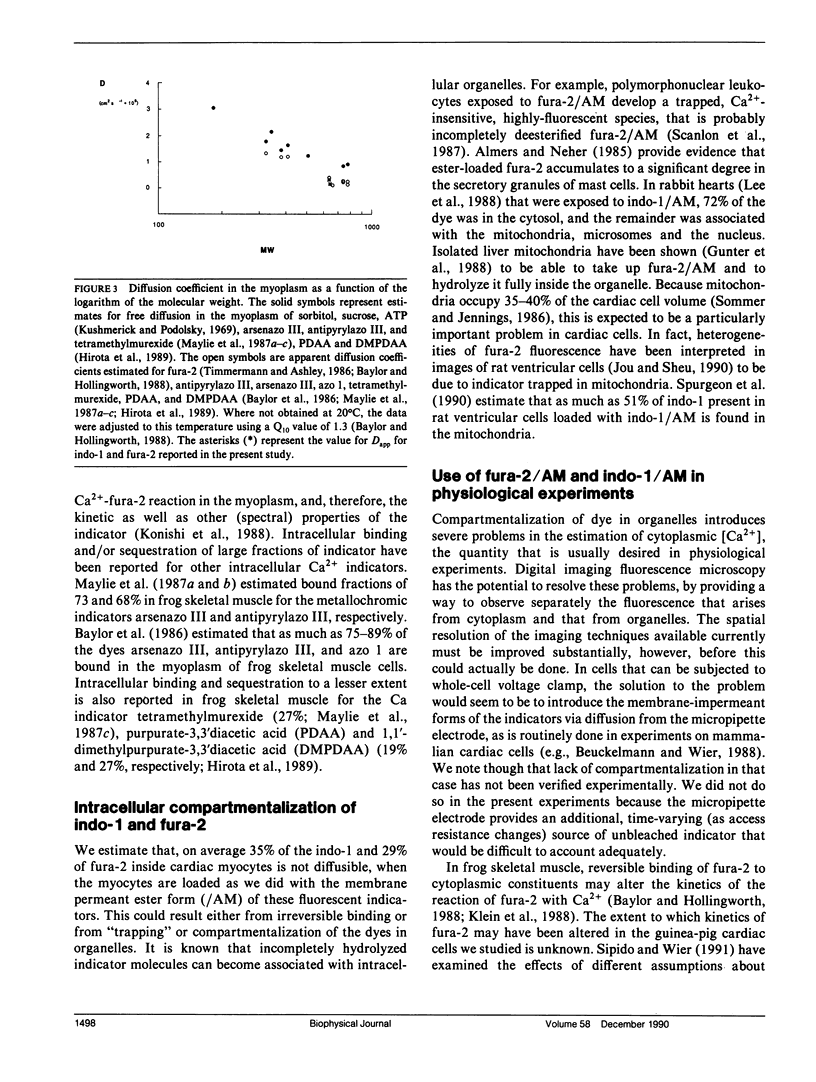
We studied intracellular binding and possible compartmentalization of the fluorescent Ca2+ indicators, indo-1 and fura-2, in single mammalian cardiac ventricular cells that had been loaded with indo-1 and fura-2 by exposure to the acetoxymethylester form of the indicators (indo-1/AM and fura-2/AM). Techniques similar to those used in experiments on fluorescence recovery after photobleaching (FRAP) were used. It was assumed that reversible binding in myoplasm would be evident as slowed recovery of fluorescence after photobleaching, and that irreversible binding of the indicators to immobile myoplasmic sites (or "compartmentalization" in organelles) would be evident as incomplete recovery. Through the use of a mask, one half of a cell was exposed to high-intensity ultraviolet (UV) light to bleach the indo-1 or fura-2 in only that part of the cell. Upon removal of the mask and termination of the high-intensity UV illumination, fluorescence recovered in the bleached half of the cell, indicating diffusion of indo-1 and fura-2. Mathematical modeling of the diffusional redistribution of the indicators indicated that in these cells the apparent diffusion coefficient for indo-1 is 1.57 x 10(-7) cm2 s-1 (SD 0.48 x 10(-7) cm2 s-1; n = 5 cells, 21 degrees C), and for fura-2 is 3.19 x 10(-7) cm2 s-1 (SD 1.85 x 10(-7) cm2 s-1; n = 6 cells, 21 degrees C). These values are approximately 6 and 3, respectively, times smaller than those expected for free diffusion in the myoplasm. In the bleached half of the cell the recovered level of fluorescence never reached the final level in the half not exposed to UV light. The extent of incomplete recovery was variable amongst the cells. Our analysis indicated that, under the conditions we used, approximately one-third of the intracellular dye is not diffusible in the myoplasm.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Hui C. S., Quinta-Ferreira M. E. Properties of the metallochromic dyes Arsenazo III, Antipyrylazo III and Azo1 in frog skeletal muscle fibres at rest. J Physiol. 1986 Aug;377:89–141. doi: 10.1113/jphysiol.1986.sp016178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gunter T. E., Restrepo D., Gunter K. K. Conversion of esterified fura-2 and indo-1 to Ca2+-sensitive forms by mitochondria. Am J Physiol. 1988 Sep;255(3 Pt 1):C304–C310. doi: 10.1152/ajpcell.1988.255.3.C304. [DOI] [PubMed] [Google Scholar]
- Hirota A., Chandler W. K., Southwick P. L., Waggoner A. S. Calcium signals recorded from two new purpurate indicators inside frog cut twitch fibers. J Gen Physiol. 1989 Oct;94(4):597–631. doi: 10.1085/jgp.94.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Tsien R. Y. Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump relaxation measurements. Biophys J. 1988 Apr;53(4):635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Simon B. J., Szucs G., Schneider M. F. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophys J. 1988 Jun;53(6):971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. High temporal resolution video imaging of intracellular calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):111–120. doi: 10.1016/0143-4160(90)90064-2. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]