Abstract
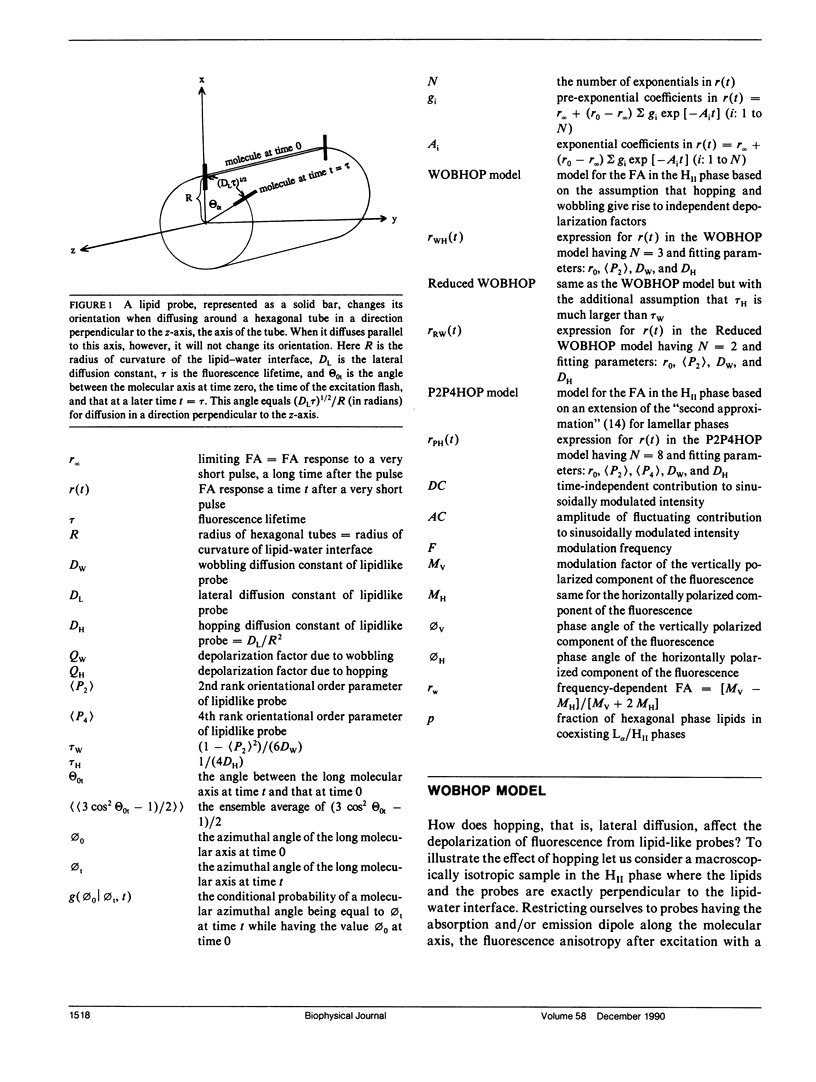
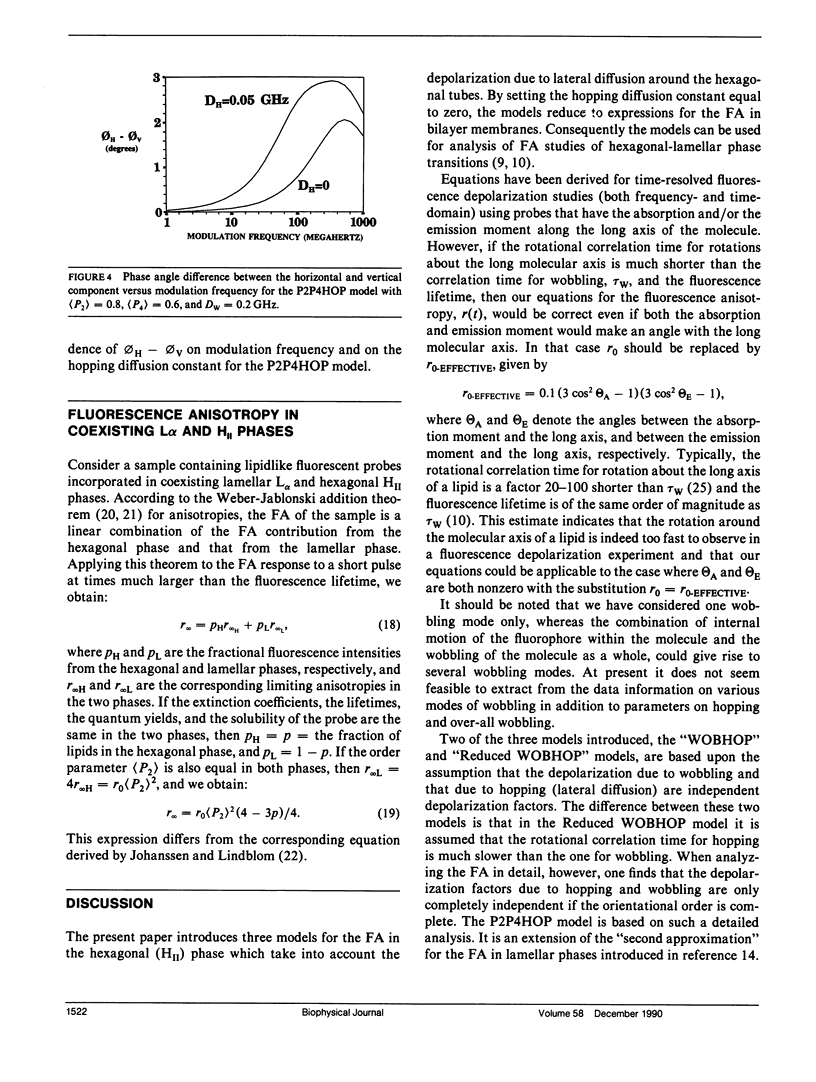
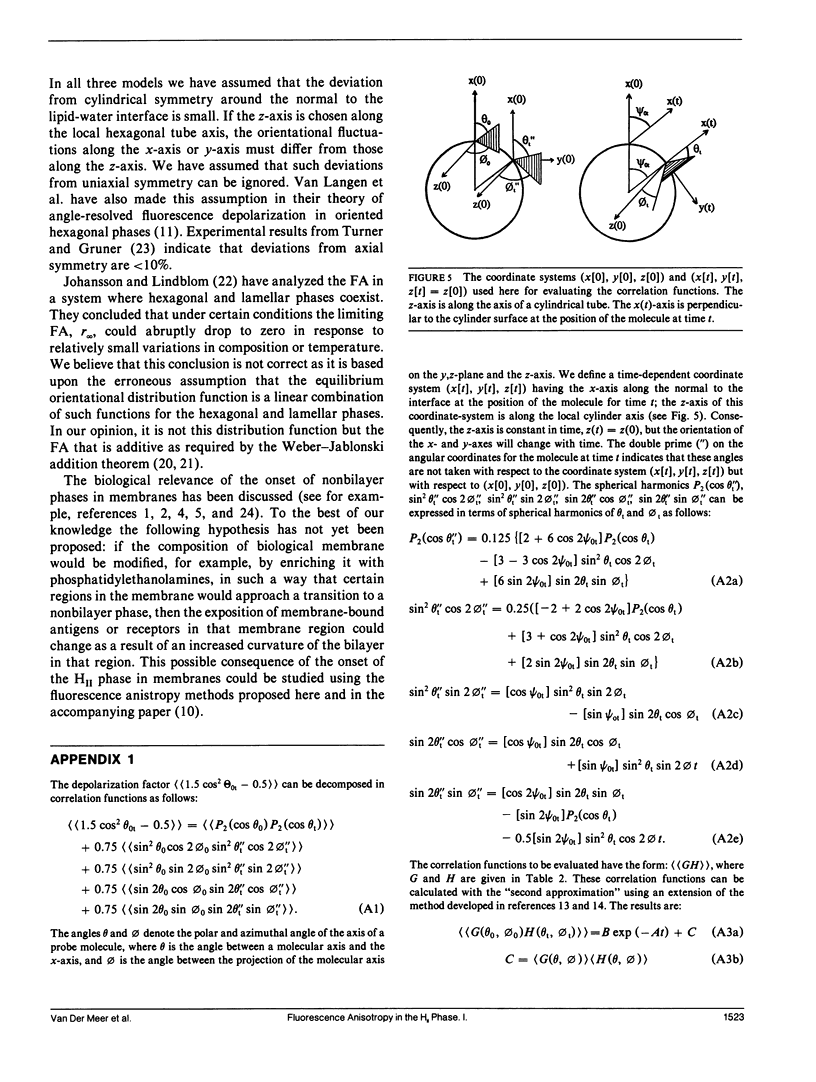
It is shown that fluorescence anisotropy from lipidlike probes in the hexagonal HII phase gives information of (a) orientational order parameters, (b) the wobbling diffusion constant, and (c) the hopping diffusion constant of the probe, DH, equals DL/R2, the lateral diffusion constant over the square of the radius of the hexagonal tubes. Here we consider only lipidlike probes having the absorption transition movement and/or the emission transition moment along the long axis of the molecule. Three models are introduced for analysis of time-resolved data: the "WOBHOP," the "reduced WOBHOP," and the "P2P4HOP" model. The fluorescence anisotropy in response to a very short excitation pulse in each of the three models is a constant plus a number of exponentials. The WOBHOP and reduced WOBHOP models have 3 and 2 exponentials, respectively, and both contain four fitting parameters: r0 (the fundamental anisotropy), (P2) (the second rank orientational order parameter), DW (the wobbling diffusion constant), and DH (the hopping diffusion constant). The P2P4HOP model has eight exponentials and five fitting parameters: the four parameters listed above and (P4) (the fourth rank orientational order parameter). Analysis of fluorescence anisotropy data in the hexagonal HII phase using one of these models allows for obtaining the hopping diffusion constant, and, if the lateral diffusion constant is known, the radius of the hexagonal tubes. Substitution of DH = 0 in each of the three models yields an expression for the fluorescence anisotropy that is used in the literature for lamellar (L alpha or L beta) phases. The fluorescence anisotropy in coexisting L alpha/HII phases is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. H. Fluorescence depolarization study of lamellar liquid crystalline to inverted cylindrical micellar phase transition of phosphatidylethanolamine. Biophys J. 1989 Jun;55(6):1025–1031. doi: 10.1016/S0006-3495(89)82901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H., Hui S. W. Correlation between bilayer destabilization and activity enhancement by diacylglycerols in reconstituted Ca-ATPase vesicles. Arch Biochem Biophys. 1986 Jan;244(1):382–386. doi: 10.1016/0003-9861(86)90127-x. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Lepock J. R., Hui S. W., Yeagle P. L. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986 Apr 15;261(11):5081–5087. [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Ellens H., Siegel D. P., Alford D., Yeagle P. L., Boni L., Lis L. J., Quinn P. J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989 May 2;28(9):3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Hall R. D. Multifrequency phase and modulation fluorometry. Annu Rev Biophys Bioeng. 1984;13:105–124. doi: 10.1146/annurev.bb.13.060184.000541. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. K., Moscicki J. K., Freed J. H. Dynamics of phosphatidylcholine-cholesterol mixed model membranes in the liquid crystalline state. Biophys J. 1990 Mar;57(3):445–459. doi: 10.1016/S0006-3495(90)82561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]
- Van der Meer B. W. Biomembrane structure and dynamics viewed by fluorescence. Subcell Biochem. 1988;13:1–53. doi: 10.1007/978-1-4613-9359-7_1. [DOI] [PubMed] [Google Scholar]
- van Langen H., Schrama C. A., van Ginkel G., Ranke G., Levine Y. K. Order and dynamics in the lamellar L alpha and in the hexagonal HII phase. Dioleoylphosphatidylethanolamine studied with angle-resolved fluorescence depolarization. Biophys J. 1989 May;55(5):937–947. doi: 10.1016/S0006-3495(89)82892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer W., Pottel H., Herreman W., Ameloot M., Hendrickx H., Schröder H. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. A new approximate solution of the rotational diffusion equation. Biophys J. 1984 Oct;46(4):515–523. doi: 10.1016/S0006-3495(84)84049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


