Abstract
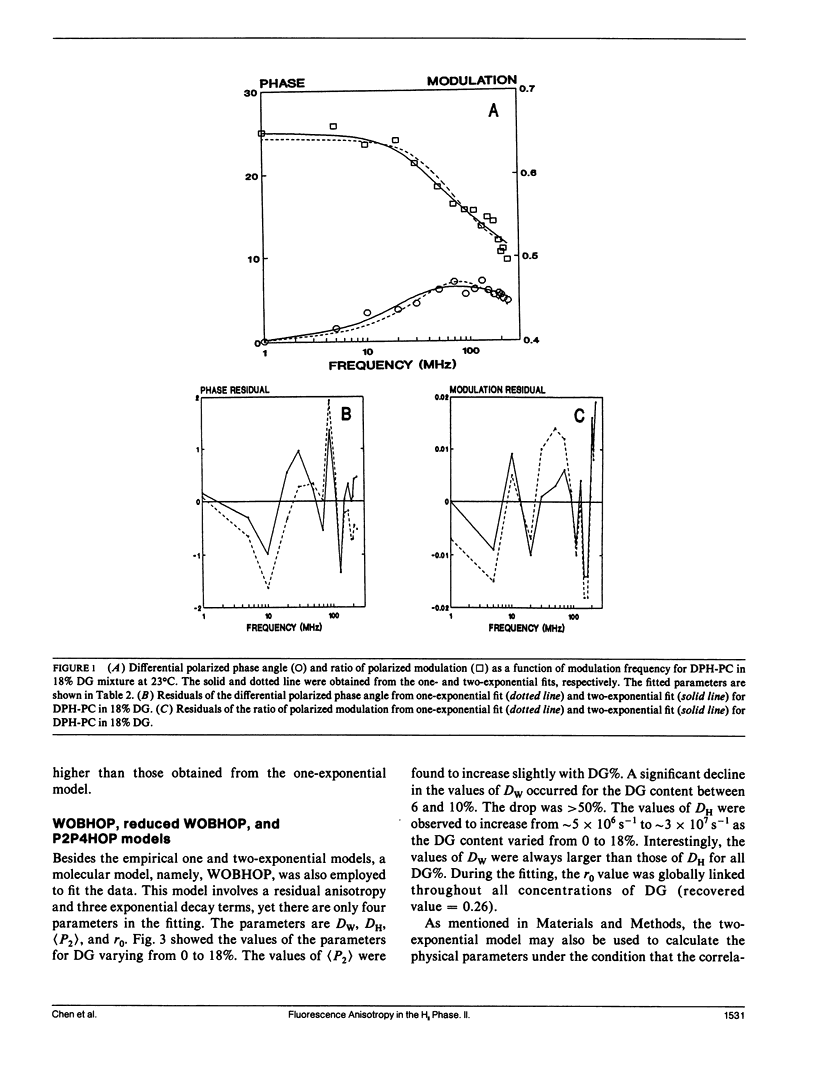
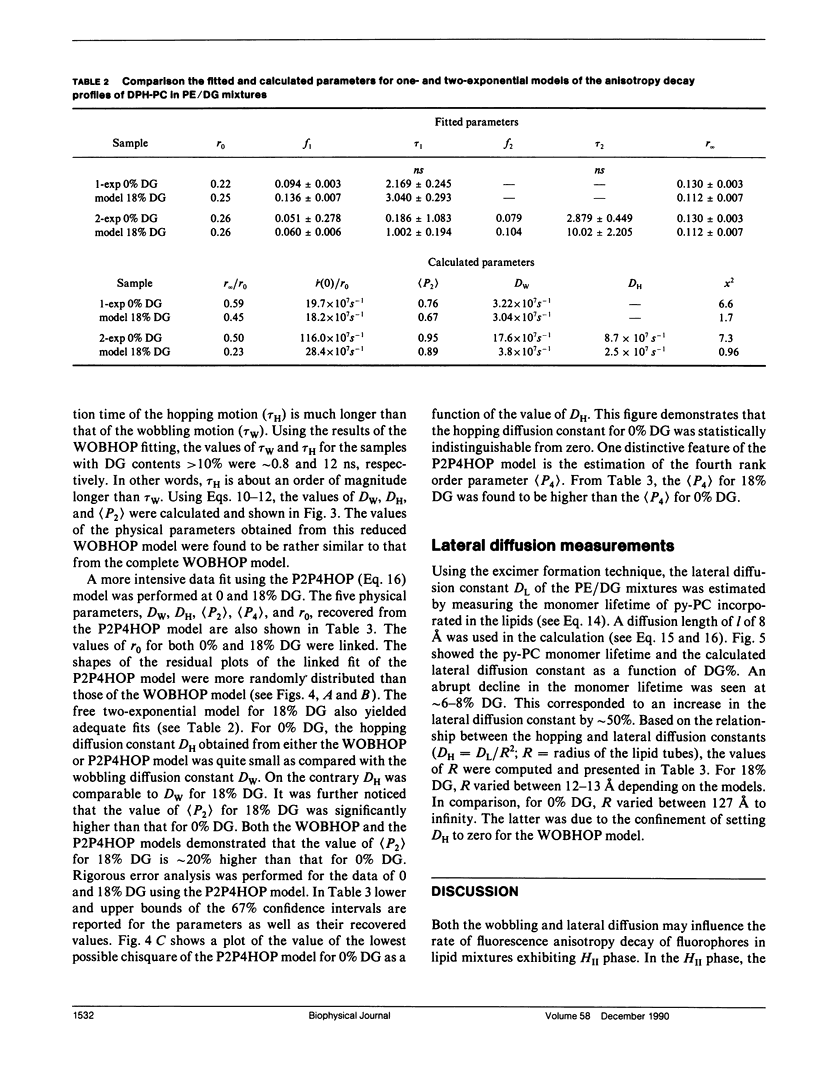
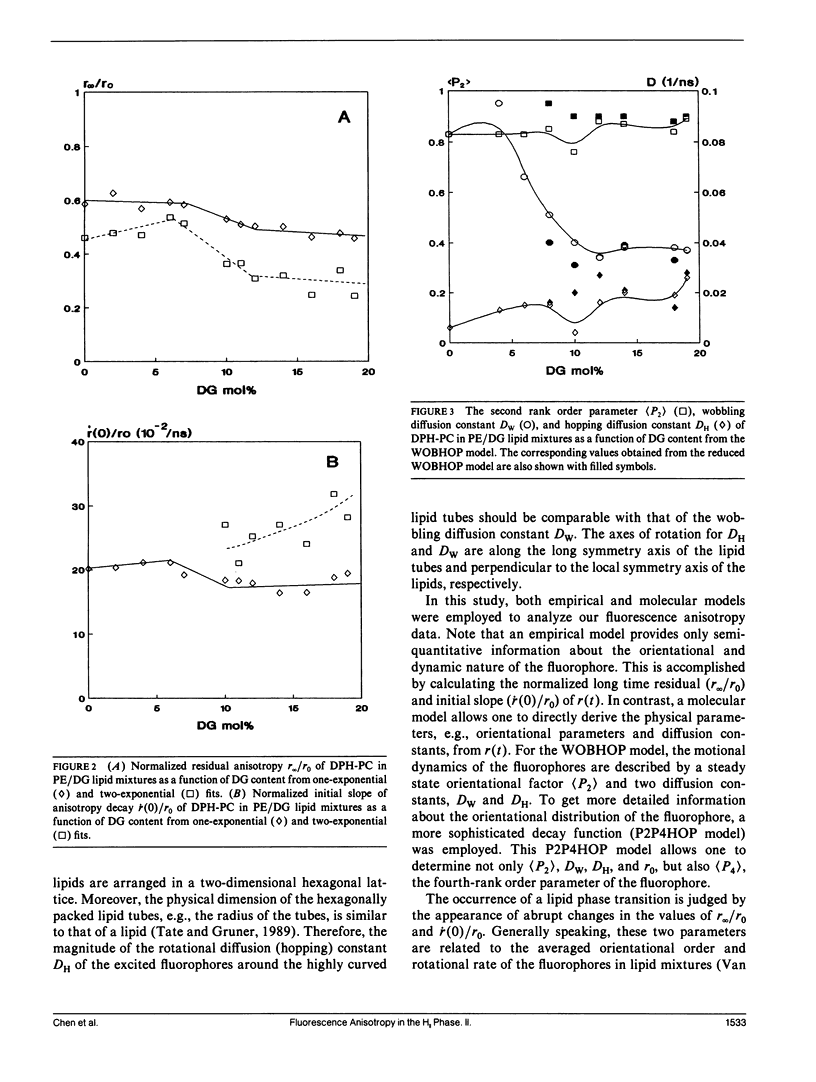
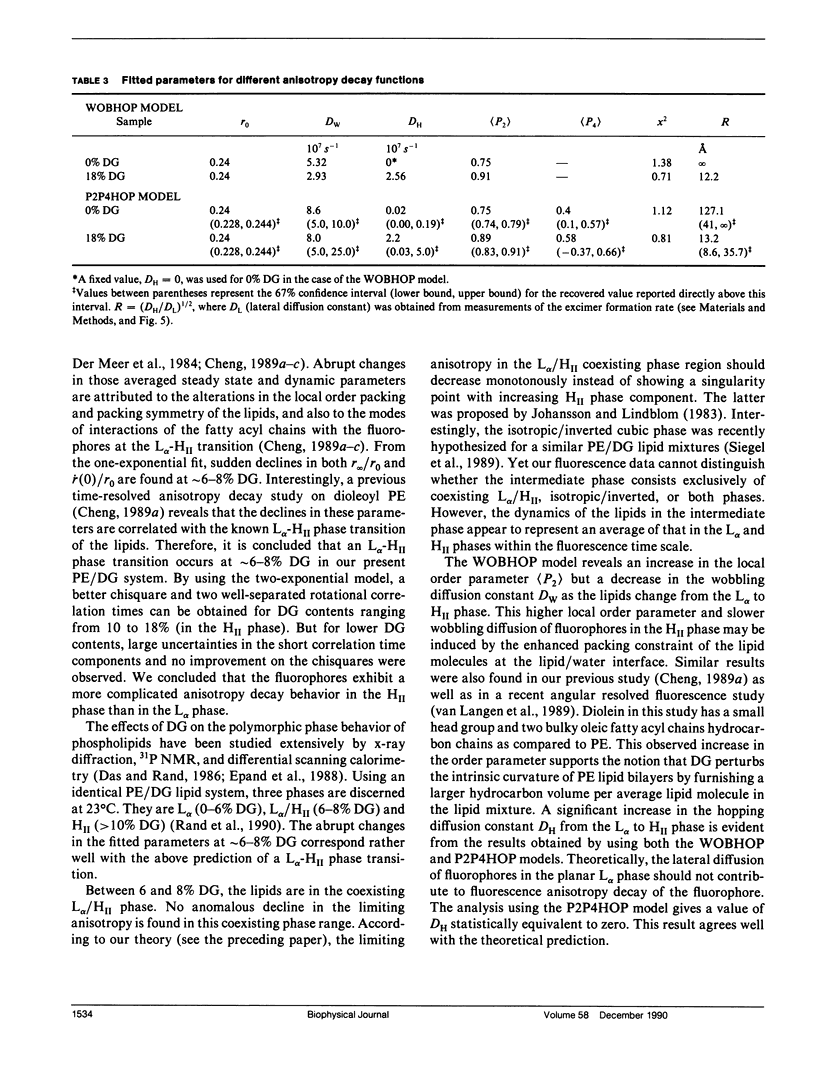
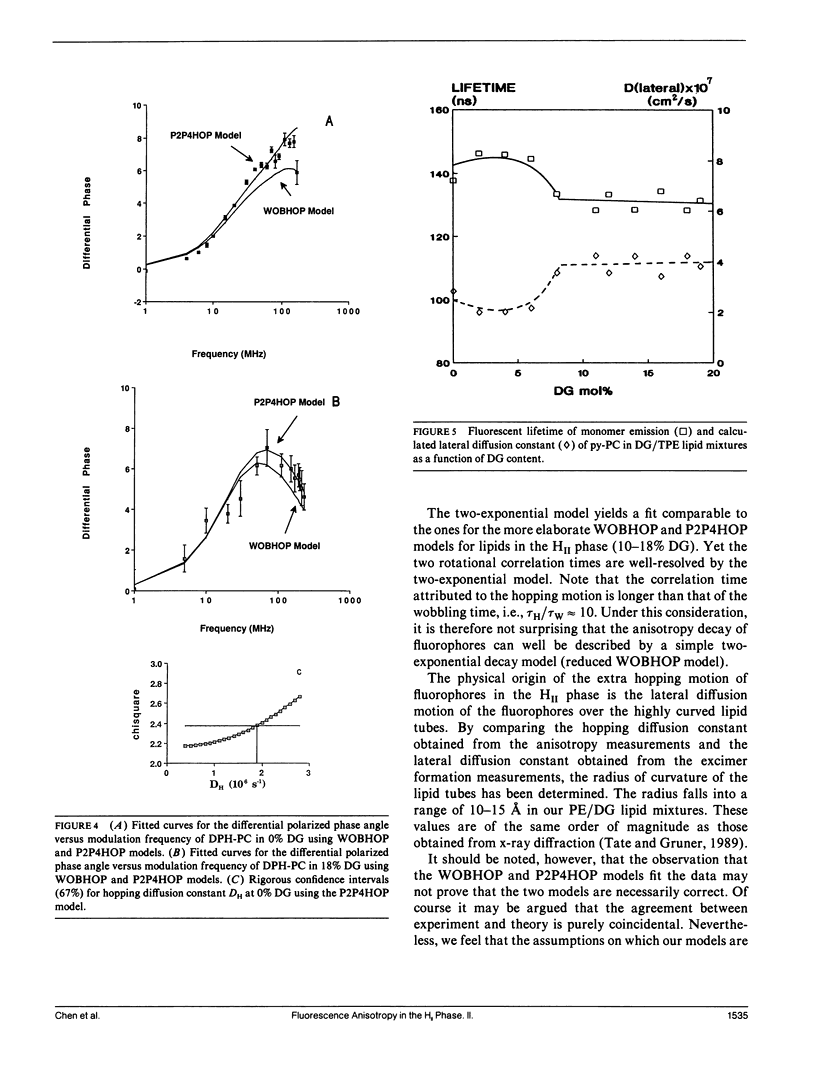
The polymorphic phase behavior of unsaturated phosphatidylethanolamine (PE)/diacylglycerol (DG) binary lipid mixtures was investigated by the use of time-resolved fluorescence anisotropy. Using a fluorescent lipid, 1-palmitoyl-2-[[2-[4-(6-phenyl-trans-1,3,5-hexatrienyl)phenylethyl] carbonyl]3-sn-phosphatidyl-choline (DPH-PC), the orientational order and rotational dynamics of the above lipid mixtures in the liquid crystalline lamellar (L alpha) and inverted hexagonal (HII) phases were studied. By employing a one-exponential model (Cheng, K.H. 1989: Biophys. J. 55:1025-1031) to fit the anisotropy decay data, abrupt decreases in the normalized initial anisotropy decay slope and the residual anisotropy of DPH-PC were observed at approximately 6-8% DG, signifying a L alpha/HII phase transition. Using our new theoretical WOBHOP and P2P4HOP models as described in a preceding paper (Van Der Meer, B.W., K.H. Cheng, and S.Y. Chen. 1990. Biophys. J. 58:000-000), two or more rotational correlation times were required to describe the anisotropy decay behavior of DPH-PC in the HII phase. These rotation correlation times were further related to the second and fourth rank order parameters, and the wobbling and hopping diffusion constants of the fluorescent probe in the highly curved lipid cylindrical tubes of the HII phase. The hopping diffusion constant (DH) equals the lateral diffusion constant (DL) divided by R2 (R = radius of the lipid cylindrical tubes). The value of DL was estimated by measuring the excimer formation rate of 1-palmitoyl-2-[10-(1-pyrenl)decanoyl] phosphatidyl choline (py-PC) in the same PE/DG mixtures. Upon comparing the values of DH and DL, the value of R was determined to be approximately 10-15 A, and agreed with that derived from x-ray diffraction (Tate, M.W., and S.M. Gruner, 1989, Biochemistry. 28:4245-4253; Rand, R.P., N.L. Fuller, S.M. Gruner, and V.A. Parsegian. 1990. Biochemistry. 29:76-87).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechem J. M., Haas E. Simultaneous determination of intramolecular distance distributions and conformational dynamics by global analysis of energy transfer measurements. Biophys J. 1989 Jun;55(6):1225–1236. doi: 10.1016/S0006-3495(89)82918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H. Fluorescence depolarization study of lamellar liquid crystalline to inverted cylindrical micellar phase transition of phosphatidylethanolamine. Biophys J. 1989 Jun;55(6):1025–1031. doi: 10.1016/S0006-3495(89)82901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H. Fluorescence depolarization study on non-bilayer phases of phosphatidylethanolamine and phosphatidylcholine lipid mixtures. Chem Phys Lipids. 1989 Oct;51(2):137–145. doi: 10.1016/0009-3084(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Hui S. W. Correlation between bilayer destabilization and activity enhancement by diacylglycerols in reconstituted Ca-ATPase vesicles. Arch Biochem Biophys. 1986 Jan;244(1):382–386. doi: 10.1016/0003-9861(86)90127-x. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Lepock J. R., Hui S. W., Yeagle P. L. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986 Apr 15;261(11):5081–5087. [PubMed] [Google Scholar]
- Chong P. L., Thompson T. E. Oxygen quenching of pyrene-lipid fluorescence in phosphatidylcholine vesicles. A probe for membrane organization. Biophys J. 1985 May;47(5):613–621. doi: 10.1016/S0006-3495(85)83957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Ellens H., Siegel D. P., Alford D., Yeagle P. L., Boni L., Lis L. J., Quinn P. J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989 May 2;28(9):3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., Lancaster C. R. Modulation of the bilayer to hexagonal phase transition of phosphatidylethanolamines by acylglycerols. Biochim Biophys Acta. 1988 Nov 22;945(2):161–166. doi: 10.1016/0005-2736(88)90478-6. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Hall R. D. Multifrequency phase and modulation fluorometry. Annu Rev Biophys Bioeng. 1984;13:105–124. doi: 10.1146/annurev.bb.13.060184.000541. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P. Construction and performance of a variable-frequency phase-modulation fluorometer. Biophys Chem. 1985 Jan;21(1):61–78. doi: 10.1016/0301-4622(85)85007-9. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Advantages and limitations of 1-palmitoyl-2-[[2-[4- (6-phenyl-trans-1,3,5-hexatrienyl)phenyl]ethyl]carbonyl]-3- sn-phosphatidylcholine as a fluorescent membrane probe. Biochemistry. 1985 Oct 22;24(22):6178–6185. doi: 10.1021/bi00343a022. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Straume M., Litman B. J. Equilibrium and dynamic structure of large, unilamellar, unsaturated acyl chain phosphatidylcholine vesicles. Higher order analysis of 1,6-diphenyl-1,3,5-hexatriene and 1-[4-(trimethylammonio)phenyl]- 6-phenyl-1,3,5-hexatriene anisotropy decay. Biochemistry. 1987 Aug 11;26(16):5113–5120. doi: 10.1021/bi00390a033. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry. 1989 May 16;28(10):4245–4253. doi: 10.1021/bi00436a019. [DOI] [PubMed] [Google Scholar]
- van Langen H., Schrama C. A., van Ginkel G., Ranke G., Levine Y. K. Order and dynamics in the lamellar L alpha and in the hexagonal HII phase. Dioleoylphosphatidylethanolamine studied with angle-resolved fluorescence depolarization. Biophys J. 1989 May;55(5):937–947. doi: 10.1016/S0006-3495(89)82892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer W., Pottel H., Herreman W., Ameloot M., Hendrickx H., Schröder H. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. A new approximate solution of the rotational diffusion equation. Biophys J. 1984 Oct;46(4):515–523. doi: 10.1016/S0006-3495(84)84049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


