Abstract
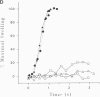
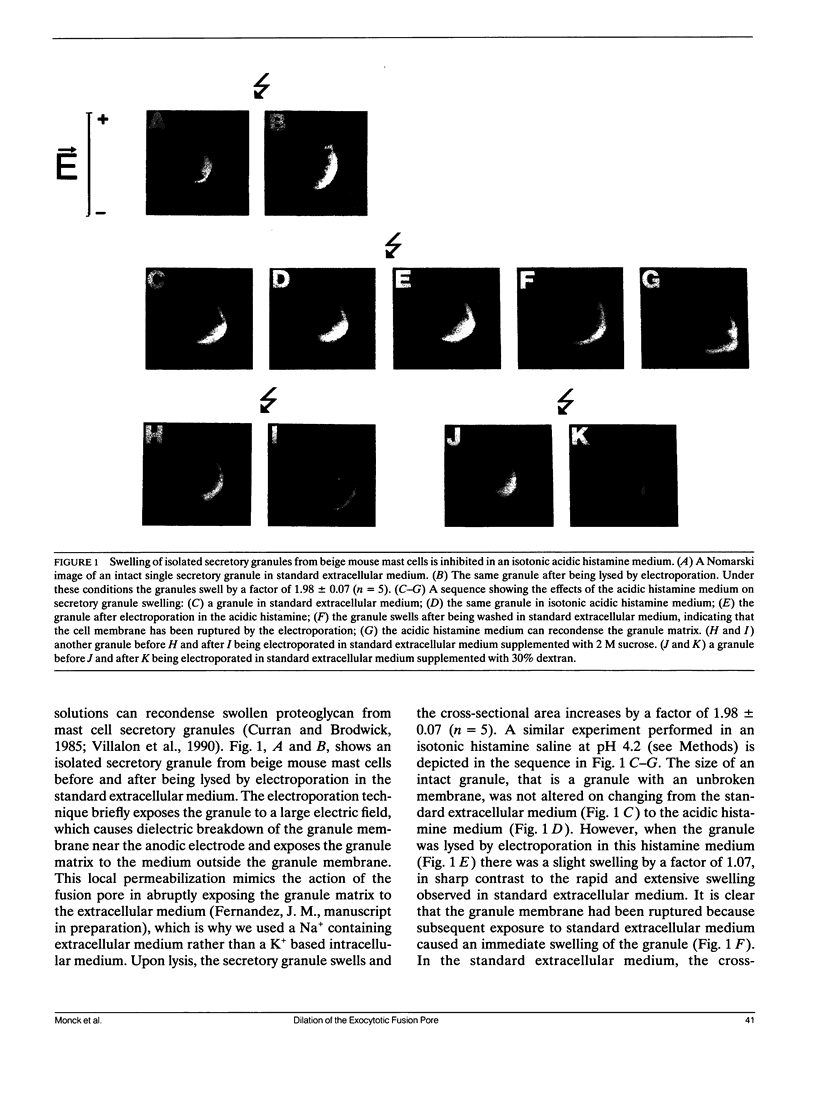
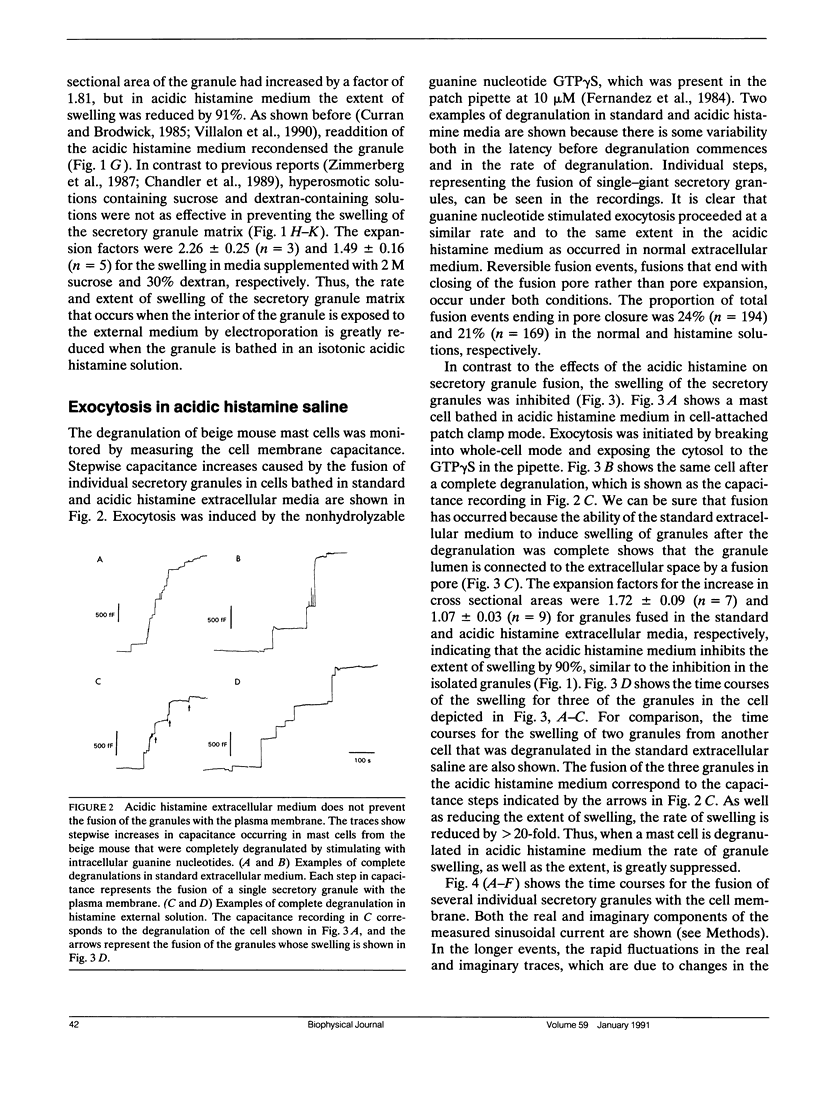
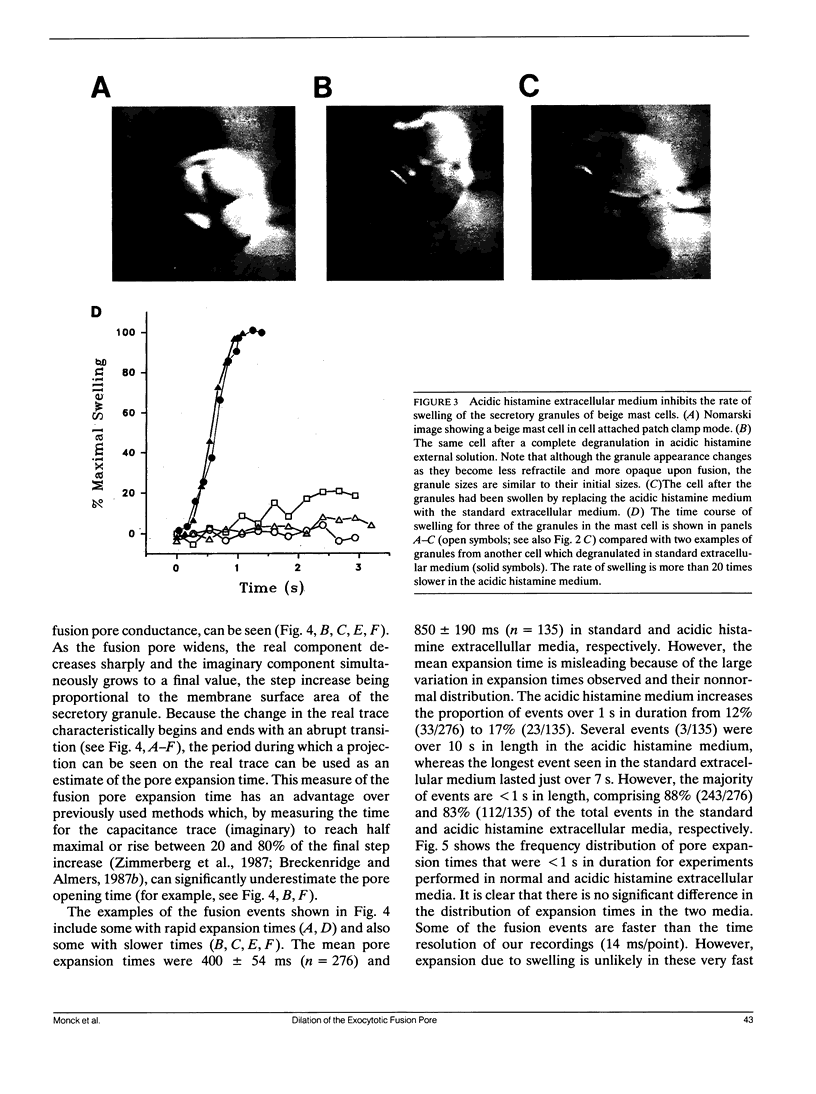
The swelling of the secretory granule matrix which follows fusion has been proposed as the driving force for the rapid expansion of the fusion pore necessary for exocytosis. To test this hypothesis, we have combined simultaneous measurements of secretory granule swelling using videomicroscopy with patch clamp measurements of the time course of the exocytotic fusion pore in mast cells from the beige mouse. We show that isotonic acidic histamine solutions are able to inhibit swelling of the secretory granule matrix both in purified secretory granules lysed by electroporation and in intact cells stimulated to exocytose by guanine nucleotides. In contrast to the inhibitory effects on granule swelling, the rate of expansion of the exocytotic fusion pore is unaffected. Therefore, as the rate of granule swelling was more than 20 times slower under these conditions, swelling of the secretory granule matrix due to water entry through the fusion pore cannot be the force responsible for the characteristic rapid expansion of the exocytotic fusion pore. We suggest that tension in the secretory granule membrane, which has recently been demonstrated in fused secretory granules, might be the force that drives the irreversible expansion of the fusion pore.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Alter S. C., Schwartz L. B. Effect of histamine and divalent cations on the activity and stability of tryptase from human mast cells. Biochim Biophys Acta. 1989 Jun 27;991(3):426–430. doi: 10.1016/0304-4165(89)90068-8. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez J. M. Patch-clamp measurements reveal multimodal distribution of granule sizes in rat mast cells. J Cell Biol. 1990 Apr;110(4):1033–1039. doi: 10.1083/jcb.110.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Bennett J. P., Gomperts B. Freeze-fracture studies of chemotactic peptide-induced exocytosis in neutrophils: evidence for two patterns of secretory granule fusion. J Ultrastruct Res. 1983 Feb;82(2):221–232. doi: 10.1016/s0022-5320(83)90055-2. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Heuser J. E. Arrest of membrane fusion events in mast cells by quick-freezing. J Cell Biol. 1980 Aug;86(2):666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Whitaker M., Zimmerberg J. High molecular weight polymers block cortical granule exocytosis in sea urchin eggs at the level of granule matrix disassembly. J Cell Biol. 1989 Sep;109(3):1269–1278. doi: 10.1083/jcb.109.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Zimmerberg J., Cohen F. S. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Green D. P. Granule swelling and membrane fusion in exocytosis. J Cell Sci. 1987 Dec;88(Pt 5):547–549. doi: 10.1242/jcs.88.5.547. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Holz R. W. Effects of changes in osmolality on the stability and function of cultured chromaffin cells and the possible role of osmotic forces in exocytosis. J Cell Biol. 1983 Apr;96(4):1082–1088. doi: 10.1083/jcb.96.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm C. A., Israelachvili J. N., McGuiggan P. M. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science. 1989 Nov 17;246(4932):919–922. doi: 10.1126/science.2814514. [DOI] [PubMed] [Google Scholar]
- Hermans M. P., Henquin J. C. Is there a role for osmotic events in the exocytotic release of insulin? Endocrinology. 1986 Jul;119(1):105–111. doi: 10.1210/endo-119-1-105. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A. Effects of osmolality and ionic strength on secretion from adrenal chromaffin cells permeabilized with digitonin. J Neurochem. 1986 Jun;46(6):1835–1842. doi: 10.1111/j.1471-4159.1986.tb08502.x. [DOI] [PubMed] [Google Scholar]
- Holz R. W. The role of osmotic forces in exocytosis from adrenal chromaffin cells. Annu Rev Physiol. 1986;48:175–189. doi: 10.1146/annurev.ph.48.030186.001135. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy J. A. Osmotic phenomena in membrane fusion. Biochem Soc Trans. 1989 Aug;17(4):623–624. doi: 10.1042/bst0170623. [DOI] [PubMed] [Google Scholar]
- Merkle C. J., Chandler D. E. Hyperosmolality inhibits exocytosis in sea urchin eggs by formation of a granule-free zone and arrest of pore widening. J Membr Biol. 1989 Dec;112(3):223–232. doi: 10.1007/BF01870953. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Alvarez de Toledo G., Fernandez J. M. Tension in secretory granule membranes causes extensive membrane transfer through the exocytotic fusion pore. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7804–7808. doi: 10.1073/pnas.87.20.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malaisse W. Hypothesis: single and chain release of insulin secretory granules is related to anionic transport at exocytotic sites. Diabetes. 1980 Nov;29(11):943–944. doi: 10.2337/diab.29.11.943. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. Beginning of exocytosis captured by rapid-freezing of Limulus amebocytes. J Cell Biol. 1981 Jul;90(1):40–54. doi: 10.1083/jcb.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Scott J. H., Zinder O., Hotchkiss A. An osmotic mechanism for exocytosis from dissociated chromaffin cells. J Biol Chem. 1984 Jan 25;259(2):1114–1121. [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Iwata A., White J. M., Almers W. Patch clamp studies of single cell-fusion events mediated by a viral fusion protein. Nature. 1989 Nov 30;342(6249):555–558. doi: 10.1038/342555a0. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Zimmerberg J. Inhibition of secretory granule discharge during exocytosis in sea urchin eggs by polymer solutions. J Physiol. 1987 Aug;389:527–539. doi: 10.1113/jphysiol.1987.sp016670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S., Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Sardet C., Epel D. Exocytosis of sea urchin egg cortical vesicles in vitro is retarded by hyperosmotic sucrose: kinetics of fusion monitored by quantitative light-scattering microscopy. J Cell Biol. 1985 Dec;101(6):2398–2410. doi: 10.1083/jcb.101.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Whitaker M. Irreversible swelling of secretory granules during exocytosis caused by calcium. Nature. 1985 Jun 13;315(6020):581–584. doi: 10.1038/315581a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]