Abstract
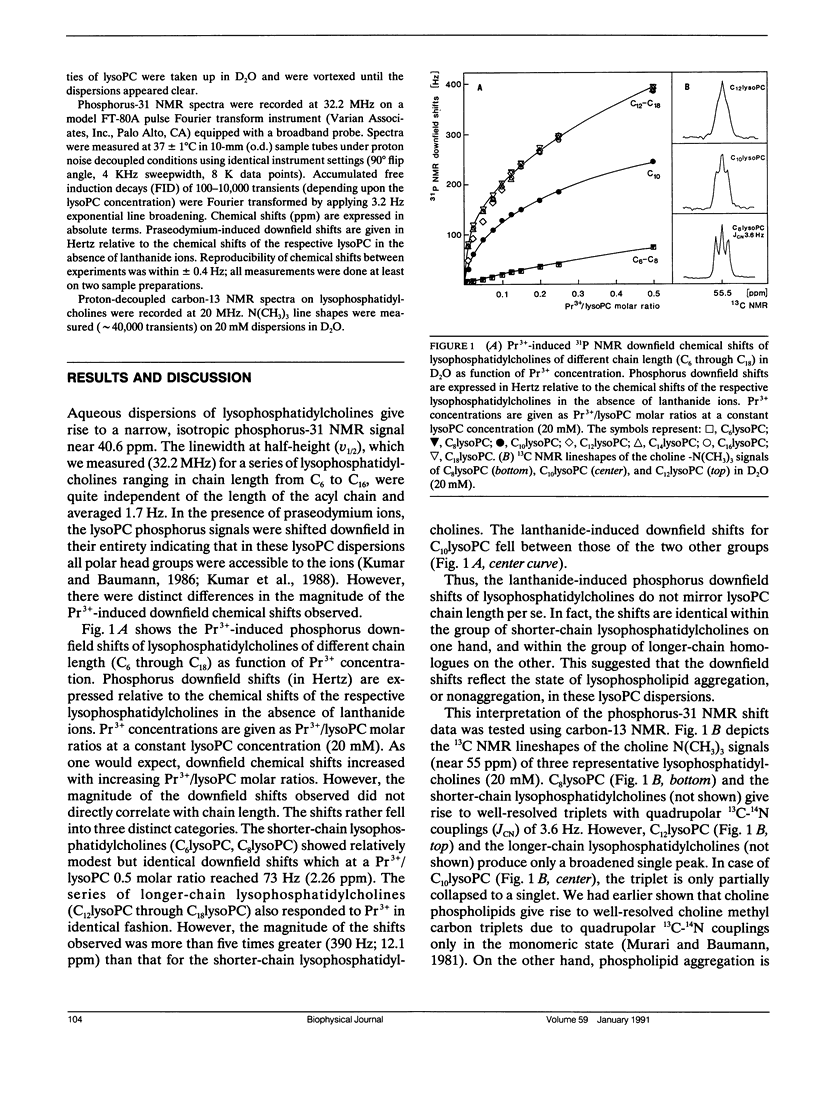
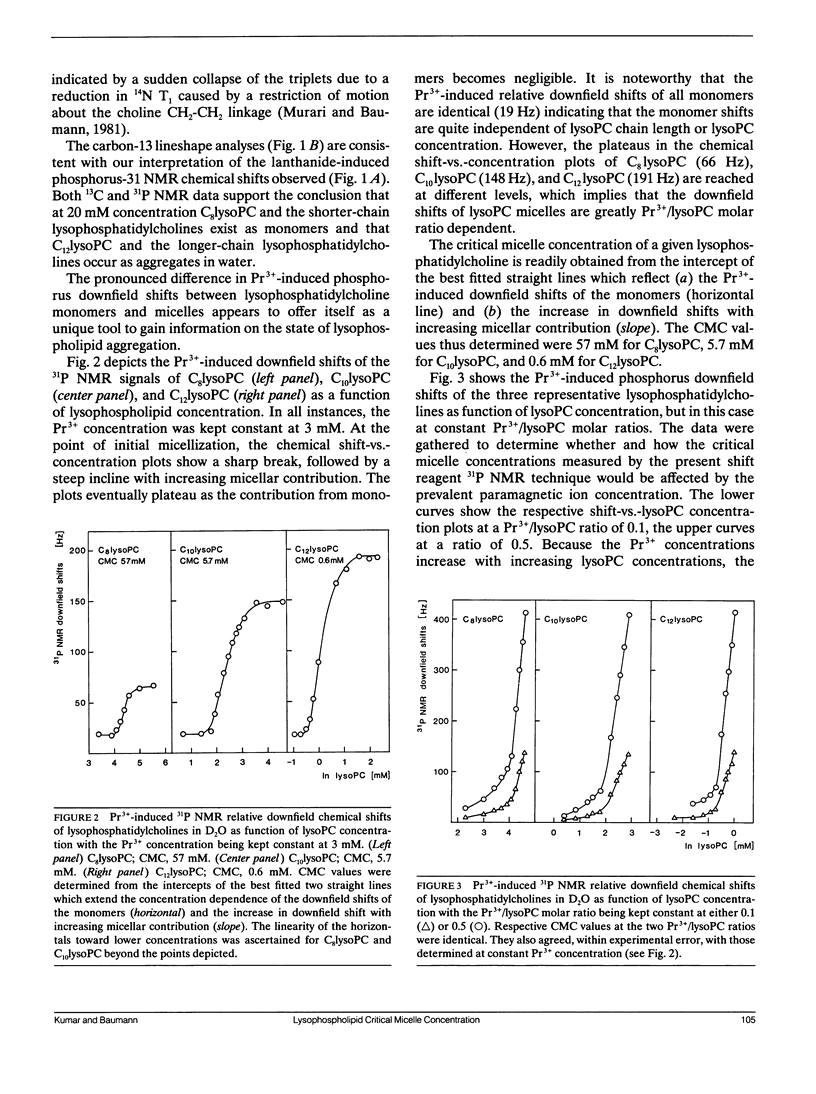
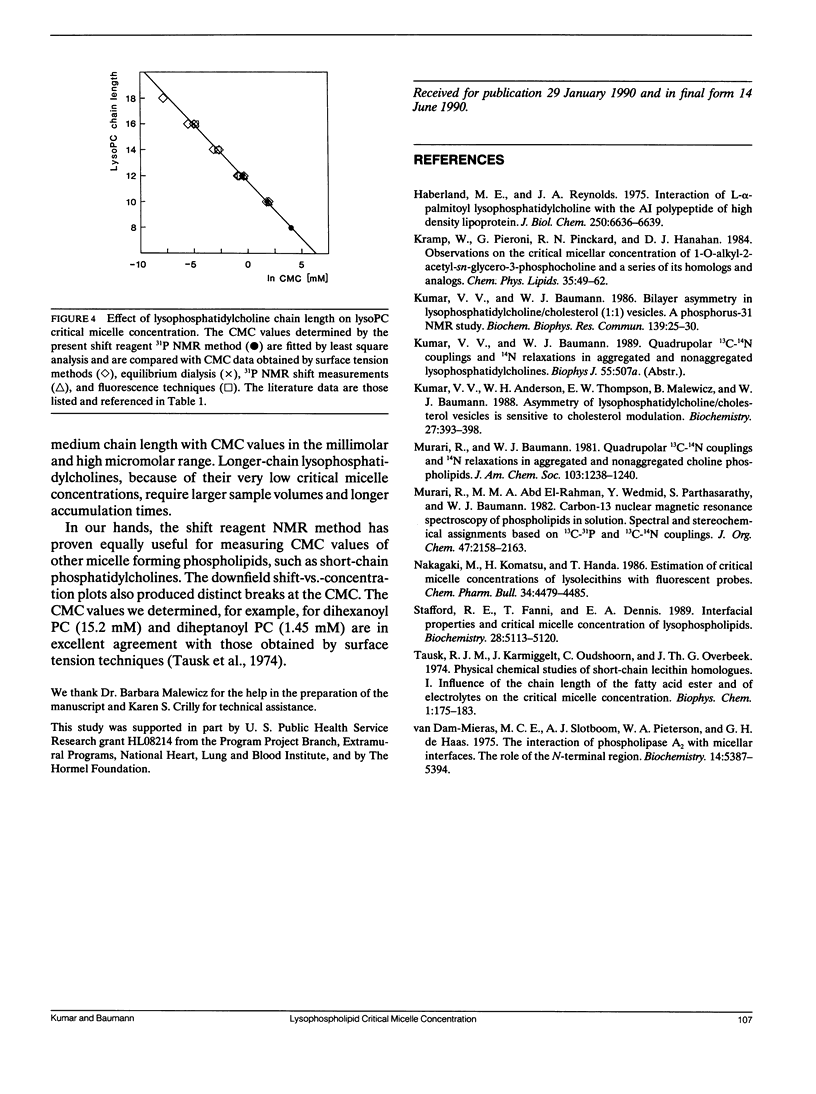
Lysophosphatidylcholine (lysoPC) monomers or micelles in water give rise to a narrow, isotropic phosphorus-31 NMR signal (40.6 ppm; v1/2 1.7 Hz; 32.2 MHz). Upon addition of praseodymium ions, the phosphorus signals are shifted downfield. However, the downfield shifts for the longer-chain lysophosphatidylcholines, which exist in the aggregated state, are far greater than those for the shorter-chain homologues, which exist as monomers. At a Pr3+/lysoPC molar ratio of 0.5, the signals of C12lysoPC through C18lysoPC were shifted by 12.1 ppm, whereas the signals of C6lysoPC and C8lysoPC were shifted by only 2.26 ppm. This very pronounced difference in lanthanide-induced downfield shifts between micelles and monomers can be utilized to determine with accuracy lysoPC critical micelle concentrations (CMC) from downfield shift-vs.-concentration plots. The CMC values we determined were 57 mM for C8lysoPC, 5.7 mM for C10lysoPC, and 0.6 mM for C12lysoPC. The shift reagent phosphorus-31 nuclear magnetic resonance technique particularly lends itself to the measurement of CMC values in the millimolar and high micromolar range. The method can equally be used for measuring critical micelle concentrations of short-chain phosphatidylcholines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haberland M. E., Reynolds J. A. Interaction of L-alpha-palmitoyl lysophosphatidylcholine with the AI polypeptide of high density lipoprotein. J Biol Chem. 1975 Sep 10;250(17):6636–6639. [PubMed] [Google Scholar]
- Kramp W., Pieroni G., Pinckard R. N., Hanahan D. J. Observations on the critical micellar concentration of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine and a series of its homologs and analogs. Chem Phys Lipids. 1984 May;35(1):49–62. doi: 10.1016/0009-3084(84)90032-x. [DOI] [PubMed] [Google Scholar]
- Kumar V. V., Anderson W. H., Thompson E. W., Malewicz B., Baumann W. J. Asymmetry of lysophosphatidylcholine/cholesterol vesicles is sensitive to cholesterol modulation. Biochemistry. 1988 Jan 12;27(1):393–398. doi: 10.1021/bi00401a059. [DOI] [PubMed] [Google Scholar]
- Kumar V. V., Baumann W. J. Bilayer asymmetry in lysophosphatidylcholine/cholesterol (1:1) vesicles. A phosphorus-31 NMR study. Biochem Biophys Res Commun. 1986 Aug 29;139(1):25–30. doi: 10.1016/s0006-291x(86)80074-2. [DOI] [PubMed] [Google Scholar]
- Stafford R. E., Fanni T., Dennis E. A. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry. 1989 Jun 13;28(12):5113–5120. doi: 10.1021/bi00438a031. [DOI] [PubMed] [Google Scholar]
- Tausk R. J., Karmiggelt J., Oudshoorn C., Overbeek J. T. Physical chemical studies of short-chain lecithin homologues. I. Influence of the chain length of the fatty acid ester and of electrolytes on the critical micelle concentration. Biophys Chem. 1974 Feb;1(3):175–183. doi: 10.1016/0301-4622(74)80004-9. [DOI] [PubMed] [Google Scholar]
- van Dam-Mieras M. C., Slotboom A. J., Pieterson W. A., de Haas G. H. The interaction of phospholipase A2 with micellar interfaces. The role of the N-terminal region. Biochemistry. 1975 Dec 16;14(25):5387–5394. doi: 10.1021/bi00696a001. [DOI] [PubMed] [Google Scholar]


