Abstract
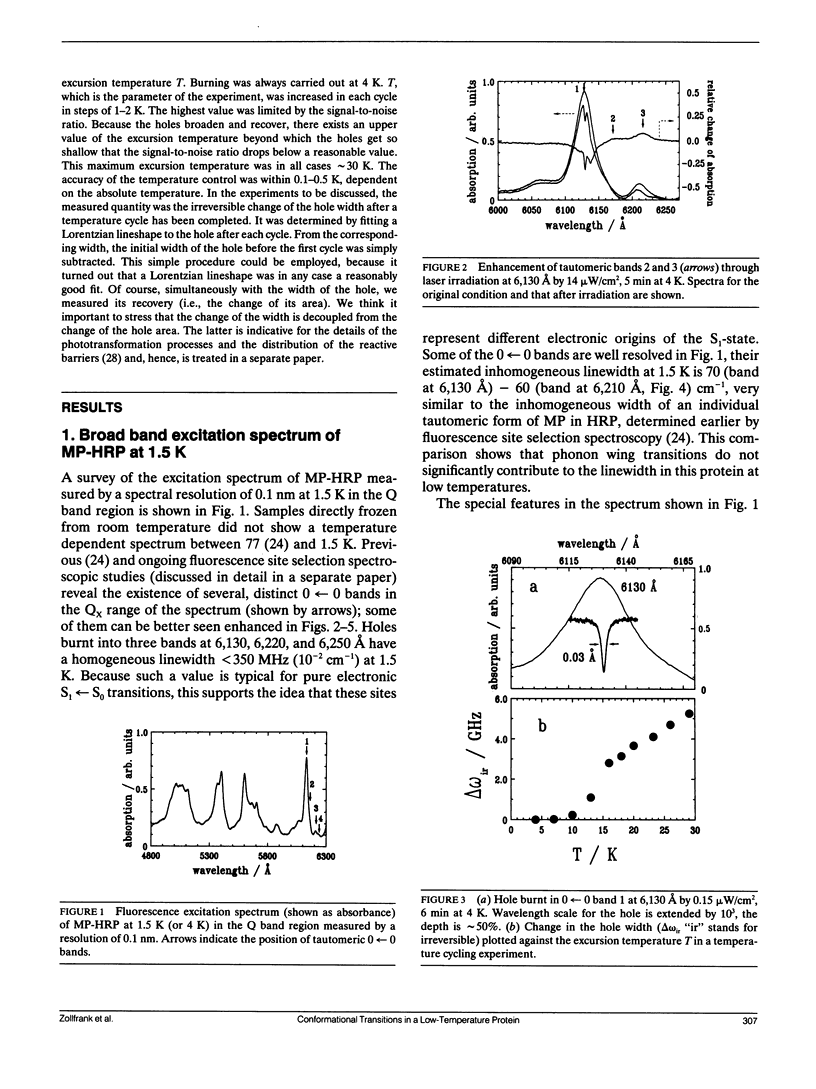
For the first time, conformational relaxation processes have been measured in a small protein, mesoporphyrin-horseradish peroxidase via their influence on spectral diffusion broadening of holes burnt in the fluorescence excitation spectrum of free base mesoporphyrin. Holes were burnt in three 0----0 bands of different tautomeric forms of the chromophore at 1.5 and 4 K, and the spectral diffusion broadening was measured in temperature cycling experiments between 4 and 30 K. The inhomogeneous linewidth for the tautomeric 0----0 bands was estimated to be 60-70 cm-1; the hole width was found narrow, being in the order of 350 MHz (10(-2) cm-1) at 1.5 K what allowed for an extremely sensitive detection of the conformational changes. Though proteins have many features in common with glasses, the spectral diffusion broadening of photochemical holes under temperature cycling conditions in mesoporphyrin horseradish peroxidase has a very different pattern as a function of temperature. Up to 12 K, the linewidth did not significantly change, then around 14 K; a steplike broadening was observed for all three tautomers, although to a different extent. The total magnitude of line broadening up to 30 K was large and also different for the tautomers. We argue that the difference between the behavior of this protein and that of glassy matrices originate from finite size effects; the protein may be characterized by a small number of TLS, and their distribution may bear discrete features.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Berendzen J., Braunstein D. Temperature-derivative spectroscopy: a tool for protein dynamics. Proc Natl Acad Sci U S A. 1990 Jan;87(1):1–5. doi: 10.1073/pnas.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance M. R., Campbell B. F., Hoover R., Friedman J. M. Myoglobin recombination at low temperature. Two phases revealed by Fourier transform infrared spectroscopy. J Biol Chem. 1987 May 25;262(15):6959–6961. [PubMed] [Google Scholar]
- Chou K. C. Low-frequency motions in protein molecules. Beta-sheet and beta-barrel. Biophys J. 1985 Aug;48(2):289–297. doi: 10.1016/S0006-3495(85)83782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K. C. Low-frequency vibrations of helical structures in protein molecules. Biochem J. 1983 Mar 1;209(3):573–580. doi: 10.1042/bj2090573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidy J., Paul K. G., Vanderkooi J. M. Differences in the binding of aromatic substrates to horseradish peroxidase revealed by fluorescence line narrowing. Biochemistry. 1989 Sep 19;28(19):7531–7541. doi: 10.1021/bi00445a006. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Köhler W, Friedrich J. Distribution of barrier heights in amorphous organic materials. Phys Rev Lett. 1987 Nov 9;59(19):2199–2202. doi: 10.1103/PhysRevLett.59.2199. [DOI] [PubMed] [Google Scholar]
- Köhler W, Zollfrank J, Friedrich J. Thermal irreversibility in optically labeled low-temperature glasses. Phys Rev B Condens Matter. 1989 Mar 15;39(8):5414–5424. doi: 10.1103/physrevb.39.5414. [DOI] [PubMed] [Google Scholar]
- Putikka WO, Huber DL. Optical linewidths and photon-echo decays of impurities in glasses. Phys Rev B Condens Matter. 1987 Aug 15;36(6):3436–3441. doi: 10.1103/physrevb.36.3436. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Moy V. T., Maniara G., Koloczek H., Paul K. G. Site-selected fluorescence spectra of porphyrin derivatives of heme proteins. Biochemistry. 1985 Dec 31;24(27):7931–7935. doi: 10.1021/bi00348a013. [DOI] [PubMed] [Google Scholar]


