Abstract
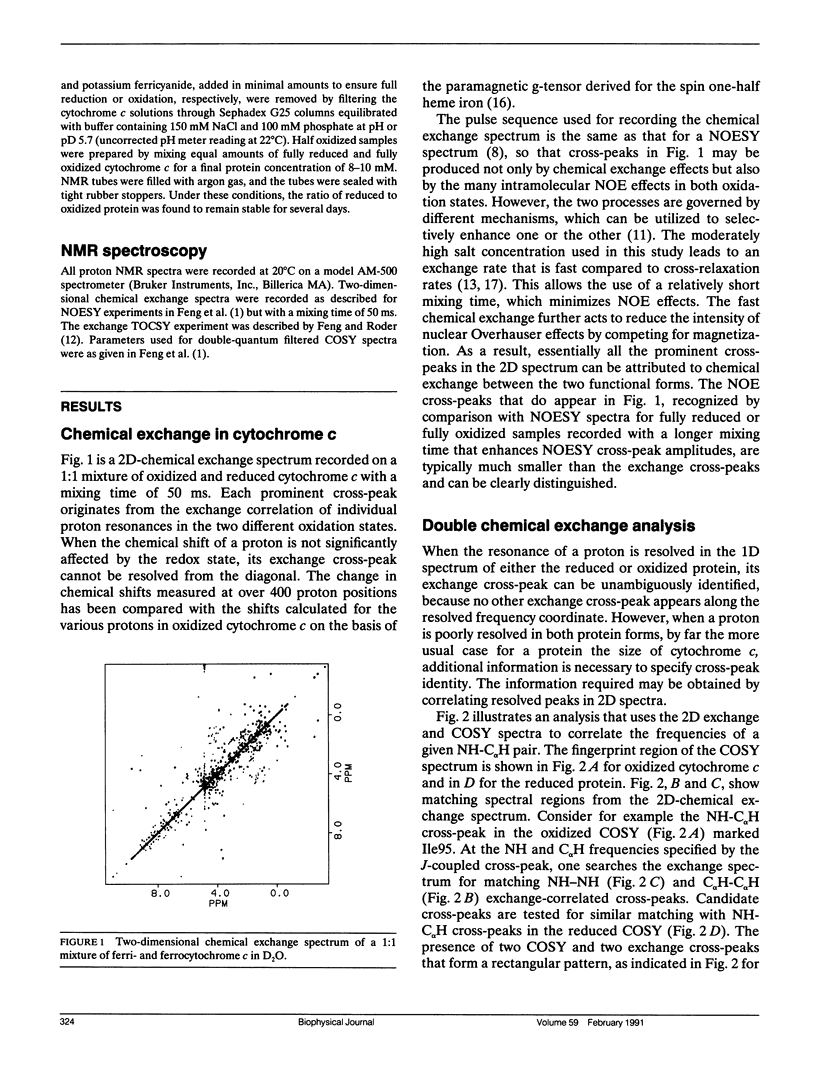
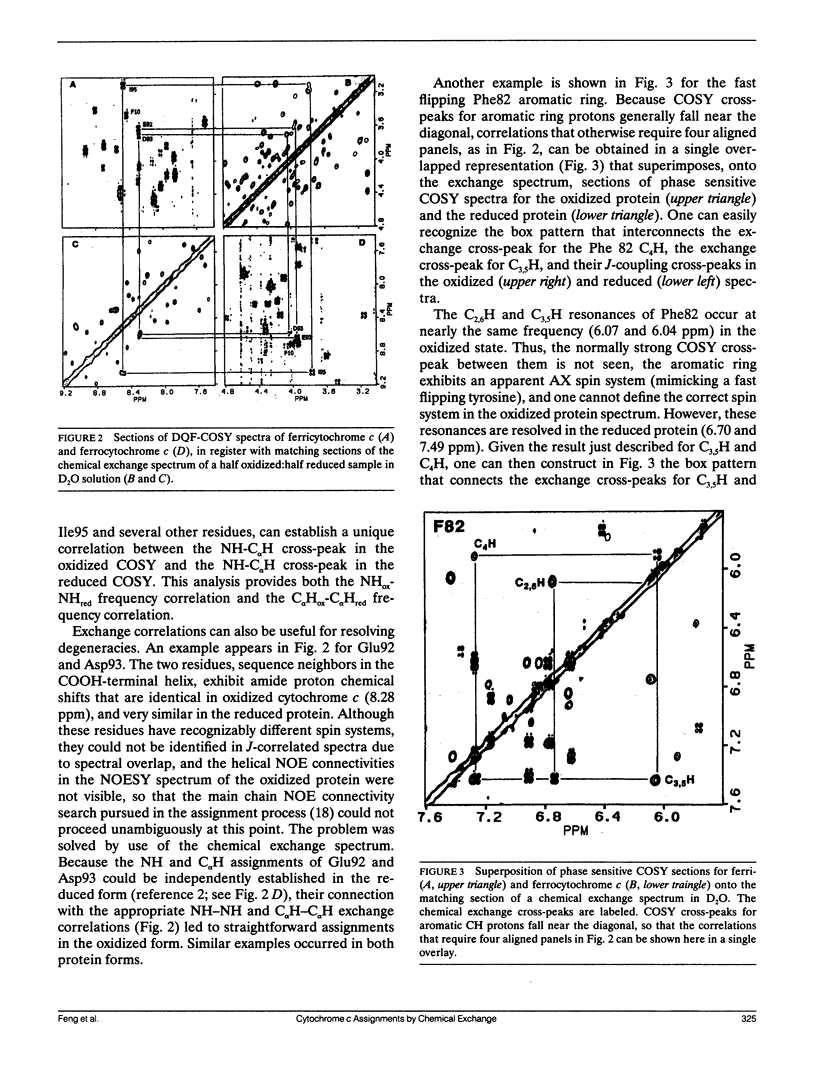
The important role played by chemical exchange in solving the proton assignment problem for oxidized and reduced horse cytochrome c is described. Some novel approaches for establishing oxidation-reduction exchange correlations in combinations of several two-dimensional spectra were used. Unambiguous chemical exchange correlations were established for 55 NH-C alpha H resonances and all the aromatic and side chain methyl resonances. Consistent although not fully unambiguous main chain proton correlations were observed for 47 of the remaining 49 residues. The many exchange correlations found serve to multiply cross-connect the two extensive, individually self-consistent networks of assignments found for the oxidized and reduced forms, and thus help to confirm both sets of assignments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boswell A. P., Moore G. R., Williams R. J., Chien J. C., Dickinson L. C. Nuclear magnetic resonance studies of the phenylalanine residues of eukaryotic cytochrome c. J Inorg Biochem. 1980 Dec;13(4):347–352. doi: 10.1016/s0162-0134(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Chazin W. J., Rance M., Wright P. E. Complete assignment of the 1H nuclear magnetic resonance spectrum of French bean plastocyanin. Application of an integrated approach to spin system identification in proteins. J Mol Biol. 1988 Aug 5;202(3):603–622. doi: 10.1016/0022-2836(88)90290-2. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Wand A. J. Main-chain-directed strategy for the assignment of 1H NMR spectra of proteins. Biochemistry. 1987 Sep 22;26(19):5953–5958. doi: 10.1021/bi00393a001. [DOI] [PubMed] [Google Scholar]
- Feng Y. Q., Roder H., Englander S. W. Assignment of paramagnetically shifted resonances in the 1H NMR spectrum of horse ferricytochrome c. Biophys J. 1990 Jan;57(1):15–22. doi: 10.1016/S0006-3495(90)82502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Roder H., Englander S. W. Redox-dependent structure change and hyperfine nuclear magnetic resonance shifts in cytochrome c. Biochemistry. 1990 Apr 10;29(14):3494–3504. doi: 10.1021/bi00466a011. [DOI] [PubMed] [Google Scholar]
- Feng Y., Roder H., Englander S. W., Wand A. J., Di Stefano D. L. Proton resonance assignments of horse ferricytochrome c. Biochemistry. 1989 Jan 10;28(1):195–203. doi: 10.1021/bi00427a027. [DOI] [PubMed] [Google Scholar]
- Griffey R. H., Redfield A. G. Proton-detected heteronuclear edited and correlated nuclear magnetic resonance and nuclear Overhauser effect in solution. Q Rev Biophys. 1987 Feb;19(1-2):51–82. doi: 10.1017/s0033583500004029. [DOI] [PubMed] [Google Scholar]
- Hammond S. J., Birdsall B., Searle M. S., Roberts G. C., Feeney J. Dihydrofolate reductase. 1H resonance assignments and coenzyme-induced conformational changes. J Mol Biol. 1986 Mar 5;188(1):81–97. doi: 10.1016/0022-2836(86)90483-3. [DOI] [PubMed] [Google Scholar]
- Keller R. M., Wüthrich K. Assignment of the heme c resonances in the 360 MHz H NMR spectra of cytochrome c. Biochim Biophys Acta. 1978 Mar 28;533(1):195–208. doi: 10.1016/0005-2795(78)90564-0. [DOI] [PubMed] [Google Scholar]
- Redfield A. G., Gupta R. K. Pulsed NMR study of the structure of cytochrome c. Cold Spring Harb Symp Quant Biol. 1972;36:405–411. doi: 10.1101/sqb.1972.036.01.052. [DOI] [PubMed] [Google Scholar]
- Redfield C., Dobson C. M. Sequential 1H NMR assignments and secondary structure of hen egg white lysozyme in solution. Biochemistry. 1988 Jan 12;27(1):122–136. doi: 10.1021/bi00401a020. [DOI] [PubMed] [Google Scholar]
- Roder H. Structural characterization of protein folding intermediates by proton magnetic resonance and hydrogen exchange. Methods Enzymol. 1989;176:446–473. doi: 10.1016/0076-6879(89)76024-9. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., Sparks S. W., Bax A. Staphylococcal nuclease: sequential assignments and solution structure. Biochemistry. 1989 Jun 27;28(13):5509–5524. doi: 10.1021/bi00439a028. [DOI] [PubMed] [Google Scholar]
- Wand A. J., Di Stefano D. L., Feng Y. Q., Roder H., Englander S. W. Proton resonance assignments of horse ferrocytochrome c. Biochemistry. 1989 Jan 10;28(1):186–194. doi: 10.1021/bi00427a026. [DOI] [PubMed] [Google Scholar]
- Williams G., Moore G. R., Porteous R., Robinson M. N., Soffe N., Williams R. J. Solution structure of mitochondrial cytochrome c. I. 1H nuclear magnetic resonance of ferricytochrome c. J Mol Biol. 1985 Jun 5;183(3):409–428. doi: 10.1016/0022-2836(85)90011-7. [DOI] [PubMed] [Google Scholar]


