Abstract
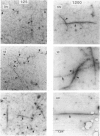
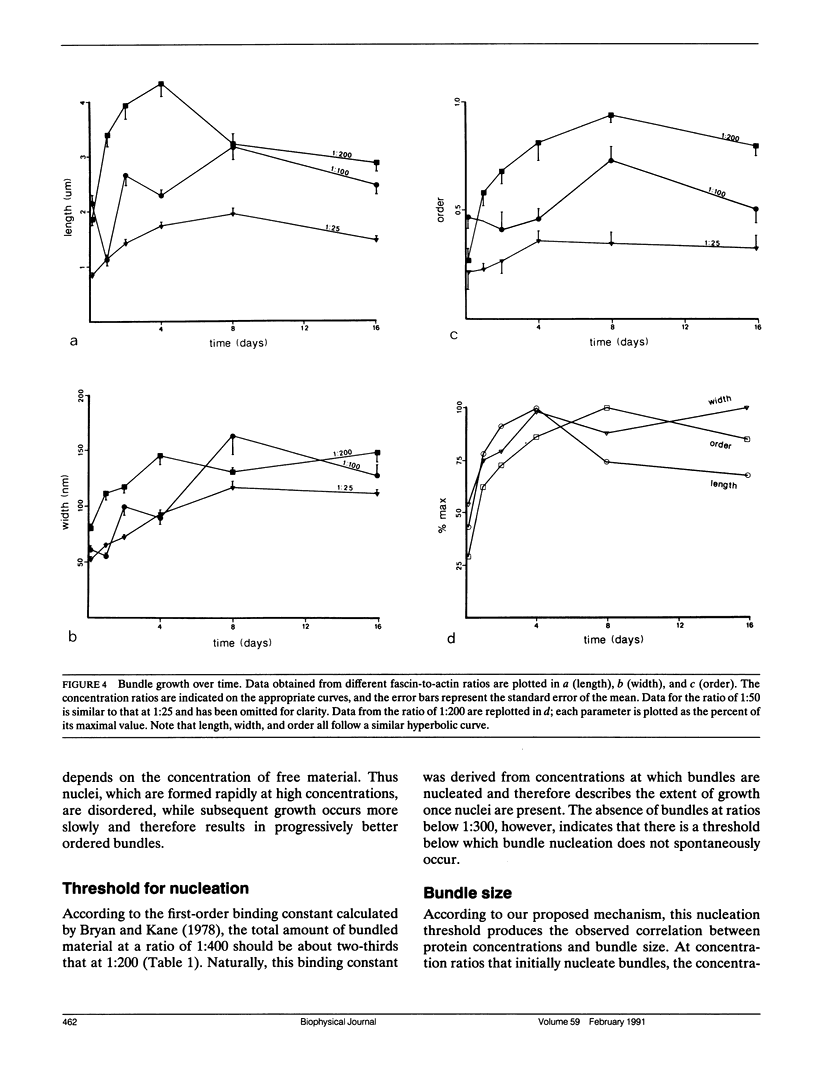
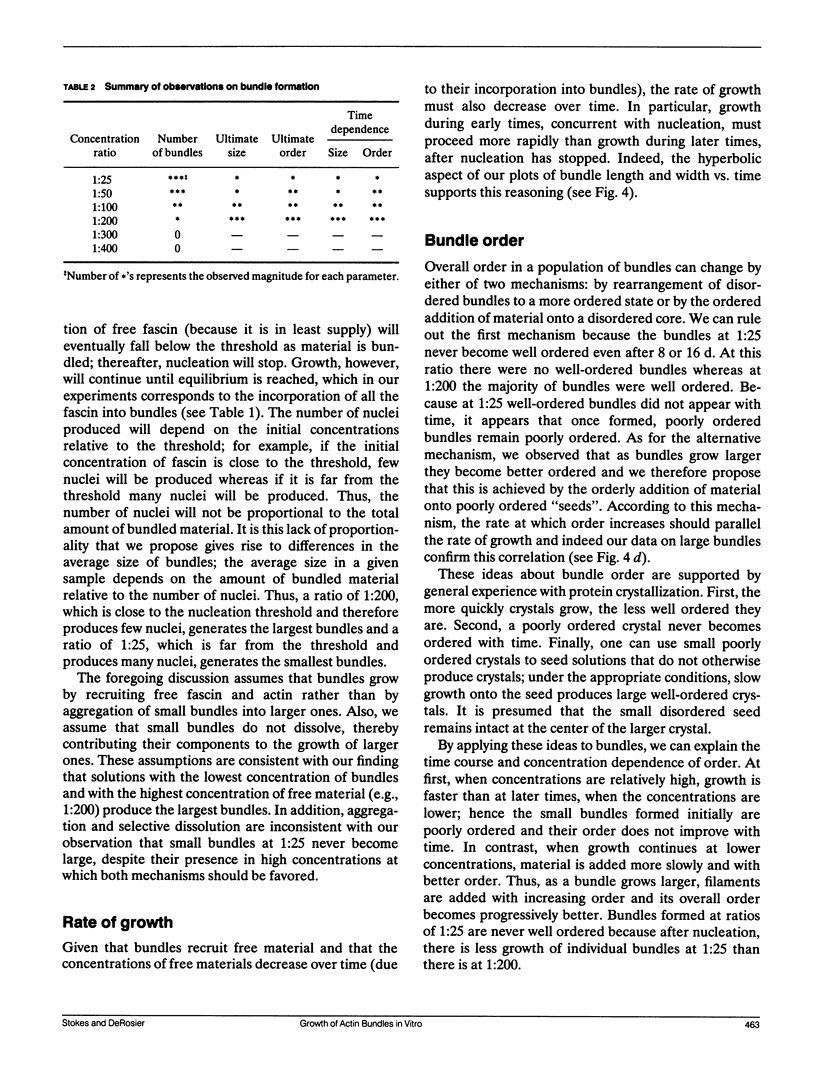
The bonding rules for actin filament bundles do not lead to a particular packing symmetry, but allow for either regular or disordered filament packing. Indeed, both hexagonal and disordered types of packing are observed in vivo. To investigate factors which control bundle order, as well as size, we examined the effect of protein concentration on the growth of actin-fascin bundles in vitro. We found that bundles require 4-8 d to achieve both maximum size and order. The largest and best ordered bundles were grown at low fascin and high actin concentrations (an initial fascin/actin ratio of 1:200). In contrast, a much larger number of poorly ordered bundles were formed at ratios of 1:25 and 1:50, and most surprisingly, no bundles were formed at 1:300 or 1:400. Based on these observations we propose a two-stage mechanism for bundle growth. The first stage is dominated by nucleation, which requires relatively high concentrations of fascin and which is therefore accompanied by rapid growth. Below some concentration threshold, nucleation ceases and bundles enter the second stage of slow growth, which continues until the supply of fascin is exhausted. By analogy with crystallization, we hypothesize that slower growth produces better order. We are able to use this mechanism to explain our observations as well as previous observations of bundle growth both in vitro and in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J., Kane R. E. Actin gelation in sea urchin egg extracts. Methods Cell Biol. 1982;25(Pt B):175–199. doi: 10.1016/s0091-679x(08)61425-9. [DOI] [PubMed] [Google Scholar]
- Bryan J., Kane R. E. Separation and interaction of the major components of sea urchin actin gel. J Mol Biol. 1978 Oct 25;125(2):207–224. doi: 10.1016/0022-2836(78)90345-5. [DOI] [PubMed] [Google Scholar]
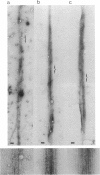
- DeRosier D. J., Censullo R. Structure of F-actin needles from extracts of sea urchin oocytes. J Mol Biol. 1981 Feb 15;146(1):77–99. doi: 10.1016/0022-2836(81)90367-3. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Tilney L. G., Egelman E. Actin in the inner ear: the remarkable structure of the stereocilium. Nature. 1980 Sep 25;287(5780):291–296. doi: 10.1038/287291a0. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Tilney L. G. How actin filaments pack into bundles. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):525–540. doi: 10.1101/sqb.1982.046.01.049. [DOI] [PubMed] [Google Scholar]
- DeRosier D., Mandelkow E., Silliman A. Structure of actin-containing filaments from two types of non-muscle cells. J Mol Biol. 1977 Jul 15;113(4):679–695. doi: 10.1016/0022-2836(77)90230-3. [DOI] [PubMed] [Google Scholar]
- Kane R. E. Induction of either contractile or structural actin-based gels in sea urchin egg cytoplasmic extract. J Cell Biol. 1980 Sep;86(3):803–809. doi: 10.1083/jcb.86.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane R. E. Preparation and purification of polymerized actin from sea urchin egg extracts. J Cell Biol. 1975 Aug;66(2):305–315. doi: 10.1083/jcb.66.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto J. J., Kane R. E., Bryan J. Redistribution of actin and fascin in sea urchin eggs after fertilization. Cell Motil. 1980;1(1):31–40. doi: 10.1002/cm.970010104. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tilney L. G., Bonder E. M., Coluccio L. M., Mooseker M. S. Actin from Thyone sperm assembles on only one end of an actin filament: a behavior regulated by profilin. J Cell Biol. 1983 Jul;97(1):112–124. doi: 10.1083/jcb.97.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J. Actin filaments, stereocilia, and hair cells of the bird cochlea. IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev Biol. 1986 Jul;116(1):119–129. doi: 10.1016/0012-1606(86)90048-5. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Egelman E. H., DeRosier D. J., Saunder J. C. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and in the cuticular plate and what happens to the organization when the stereocilia are bent. J Cell Biol. 1983 Mar;96(3):822–834. doi: 10.1083/jcb.96.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]