Abstract
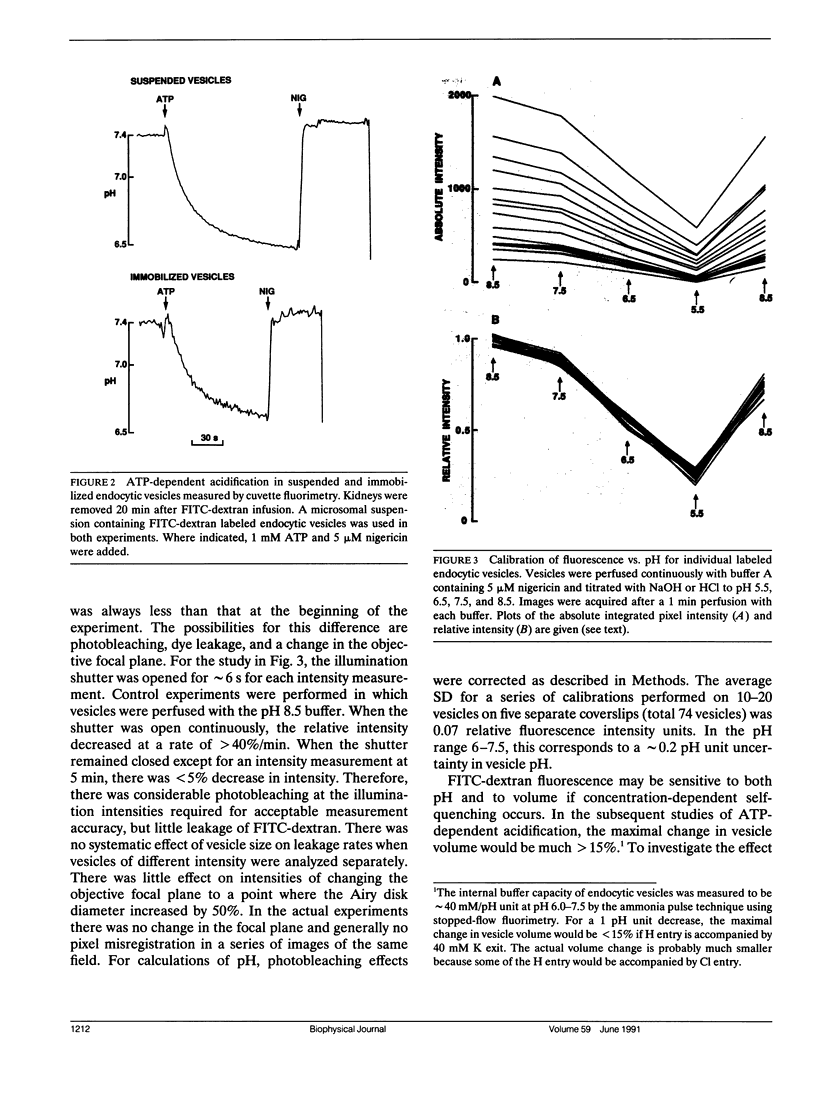
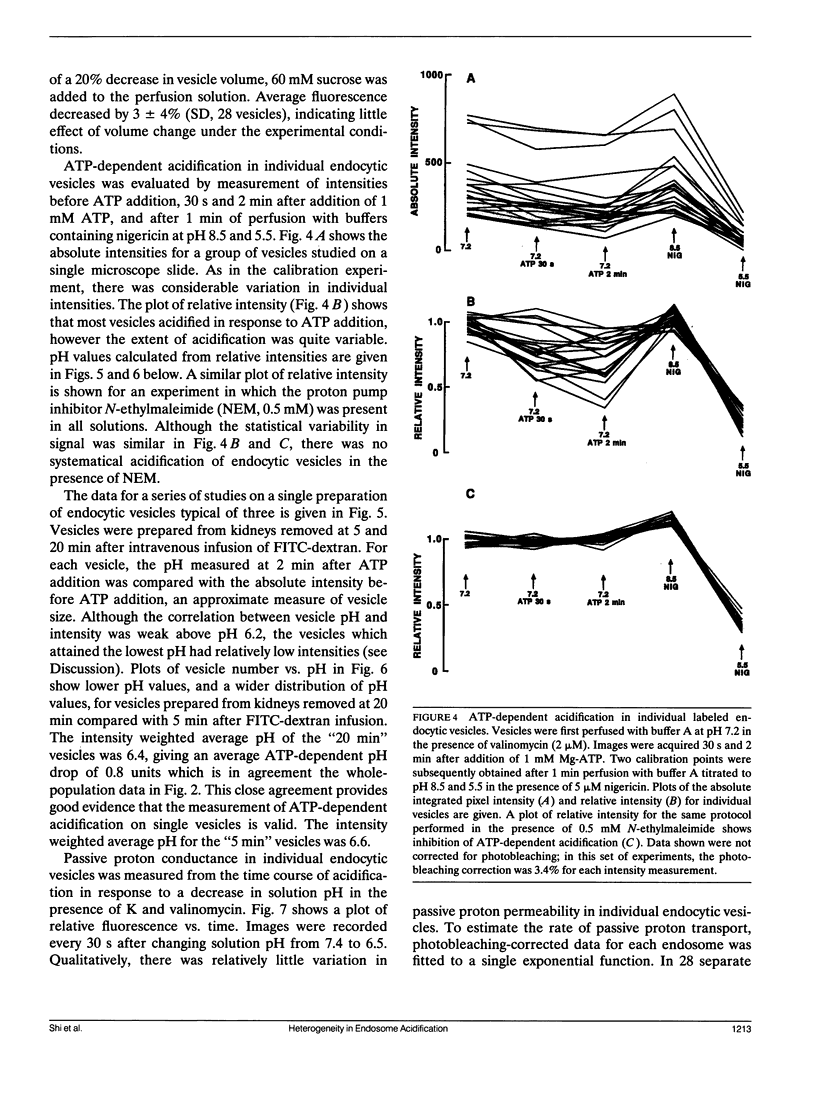
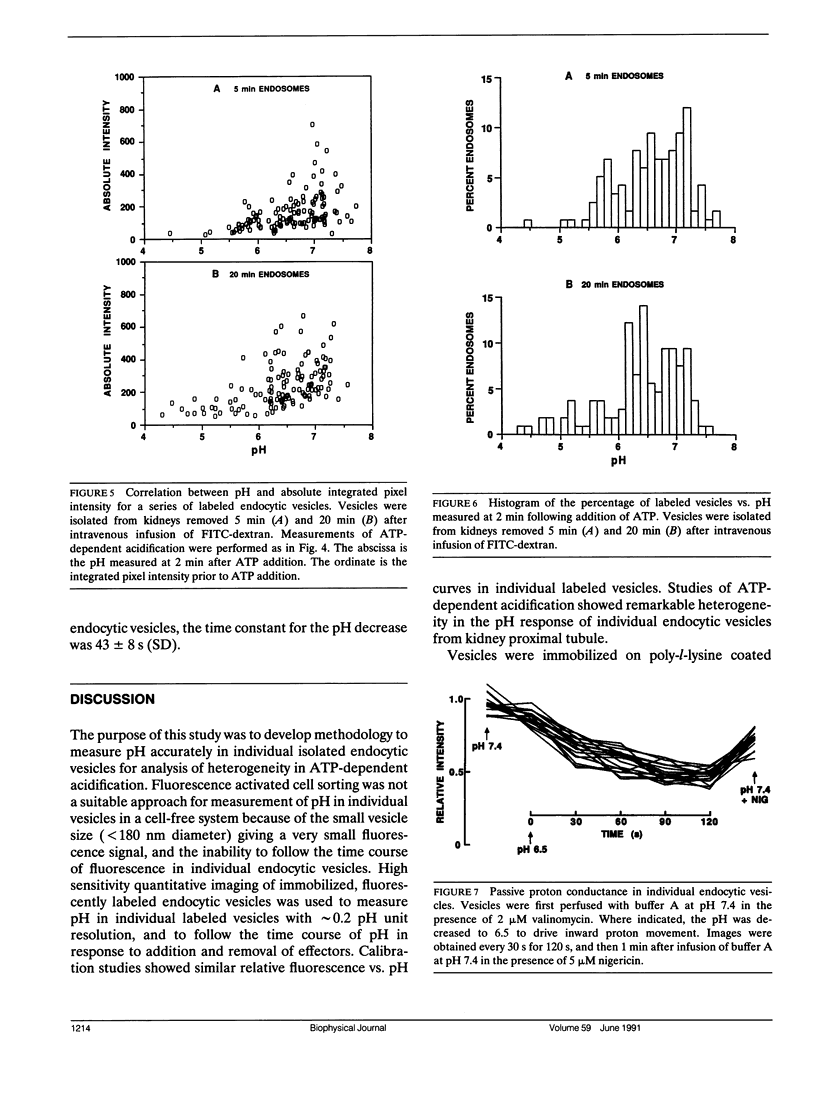
Measurement of membrane transport in suspensions of isolated membrane vesicles provides averaged information over a potentially very heterogeneous vesicle population. To examine the regulatory mechanisms for ATP-dependent acidification, methodology was developed to measure pH in individual endocytic vesicles. Endocytic vesicles from proximal tubule apical membrane of rat kidney were labeled in vivo by intravenous infusion of FITC-dextran (9 kD); a microsomal fraction was obtained from dissected renal cortex by homogenization and differential centrifugation. Vesicles were immobilized on a polylysine coated coverglass and imaged at high magnification by a silicon intensified target camera. ATP-dependent acidification was not influenced by endosome immobilization. Endosome pH was determined from the integrated fluorescence intensity of individual labeled vesicles after background subtraction. Calibration studies with high K and nigericin showed nearly identical fluorescence vs. pH curves for different endosomes with a standard deviation for a single pH measurement in a single endosome of approximately 0.2 pH units. In response to addition of 1 mM MgATP in the presence of K and valinomycin, endosome pH decreased from 7.2 to a mean of 6.4 with a unimodal distribution with width at half-maximum of approximately 1 pH unit. The drop in endosome pH increased and the shape of the distribution changed when the time between FITC-dextran infusion and kidney removal was increased from 5 to 20 min. Differences in ATP-dependent acidification could not be attributed to heterogeneity in passive proton conductance. These results establish a direct method to measure pH in single endocytic vesicles and demonstrate remarkable heterogeneity in ATP-dependent acidification which was interpreted in terms of heterogeneity in the number and/or activity of proton pumps at serial stages of endocytosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Murphy R. F. A chloroquine-resistant Swiss 3T3 cell line with a defect in late endocytic acidification. J Cell Biol. 1988 Feb;106(2):269–277. doi: 10.1083/jcb.106.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Sipe D. M., Murphy R. F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. C., Dix J. A., Sellers M. C., Verkman A. S. Fluorescence measurement of chloride transport in monolayer cultured cells. Mechanisms of chloride transport in fibroblasts. Biophys J. 1989 Dec;56(6):1071–1081. doi: 10.1016/S0006-3495(89)82755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., McGraw T. E., Maxfield F. R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989 Dec;109(6 Pt 2):3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Mâle P., Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989 Feb 5;264(4):2212–2220. [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Dix J. A., Verkman A. S. Cell membrane fluidity in the intact kidney proximal tubule measured by orientation-independent fluorescence anisotropy imaging. Biophys J. 1990 Feb;57(2):241–254. doi: 10.1016/S0006-3495(90)82527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Fuchs R., Mellman I. Analysis of endosome and lysosome acidification in vitro. Methods Enzymol. 1988;157:601–611. doi: 10.1016/0076-6879(88)57108-2. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990 Jul 26;346(6282):335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Inoué S. Imaging of unresolved objects, superresolution, and precision of distance measurement with video microscopy. Methods Cell Biol. 1989;30:85–112. doi: 10.1016/s0091-679x(08)60976-0. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Verkman A. S., Arnaout M. A., Ausiello D. A., Brown D. Endocytic vesicles from renal papilla which retrieve the vasopressin-sensitive water channel do not contain a functional H+ ATPase. J Cell Biol. 1990 Aug;111(2):379–389. doi: 10.1083/jcb.111.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W. I., Weyer P., Verkman A. S., Ausiello D. A., Brown D. FITC-dextran as a probe for endosome function and localization in kidney. Am J Physiol. 1990 Feb;258(2 Pt 1):C309–C317. doi: 10.1152/ajpcell.1990.258.2.C309. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Moriyama Y., Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci U S A. 1982 May;79(9):2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Seidman L., Farquhar M. G. The membrane composition of coated pits, microvilli, endosomes, and lysosomes is distinctive in the rat kidney proximal tubule cell. J Cell Biol. 1986 Jan;102(1):77–87. doi: 10.1083/jcb.102.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Bowser R., Murphy R. F. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol. 1987 May;131(2):200–209. doi: 10.1002/jcp.1041310209. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Characteristics of the proton pump in rat renal cortical endocytotic vesicles. Am J Physiol. 1986 May;250(5 Pt 2):F817–F826. doi: 10.1152/ajprenal.1986.250.5.F817. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Proton ATPase in rat renal cortical endocytotic vesicles. Biochim Biophys Acta. 1988 Jan 22;937(2):398–410. doi: 10.1016/0005-2736(88)90262-3. [DOI] [PubMed] [Google Scholar]
- Shi L. B., Verkman A. S. Very high water permeability in vasopressin-induced endocytic vesicles from toad urinary bladder. J Gen Physiol. 1989 Dec;94(6):1101–1115. doi: 10.1085/jgp.94.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger R. M., Papahadjopoulos D., Hong K. L. Endocytosis and intracellular fate of liposomes using pyranine as a probe. Biochemistry. 1990 May 22;29(20):4929–4939. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Proton pump-generated electrochemical gradients in rat liver multivesicular bodies. Quantitation and effects of chloride. J Biol Chem. 1988 Feb 25;263(6):2603–2611. [PubMed] [Google Scholar]
- Verkman A. S., Alpern R. J. Kinetic transport model for cellular regulation of pH and solute concentration in the renal proximal tubule. Biophys J. 1987 Apr;51(4):533–546. doi: 10.1016/S0006-3495(87)83379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A. S., Lencer W. I., Brown D., Ausiello D. A. Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature. 1988 May 19;333(6170):268–269. doi: 10.1038/333268a0. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Mechanisms and regulation of water permeability in renal epithelia. Am J Physiol. 1989 Nov;257(5 Pt 1):C837–C850. doi: 10.1152/ajpcell.1989.257.5.C837. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Takla R., Sefton B., Basbaum C., Widdicombe J. H. Quantitative fluorescence measurement of chloride transport mechanisms in phospholipid vesicles. Biochemistry. 1989 May 16;28(10):4240–4244. doi: 10.1021/bi00436a018. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Weyer P., Brown D., Ausiello D. A. Functional water channels are present in clathrin-coated vesicles from bovine kidney but not from brain. J Biol Chem. 1989 Dec 5;264(34):20608–20613. [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Endocytosis and exocytosis: current concepts of vesicle traffic in animal cells. Int Rev Cytol. 1984;92:51–92. doi: 10.1016/s0074-7696(08)61324-8. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Stone D. K., Racker E. Determinants of clathrin-coated vesicle acidification. J Biol Chem. 1983 Dec 25;258(24):14834–14838. [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2723–2733. doi: 10.1083/jcb.105.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Kinetics of endosome acidification in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2713–2721. doi: 10.1083/jcb.105.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R. G., Shi L. B., Lencer W. I., Verkman A. S. Functional colocalization of water channels and proton pumps in endosomes from kidney proximal tubule. J Gen Physiol. 1989 May;93(5):885–902. doi: 10.1085/jgp.93.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]



