Abstract
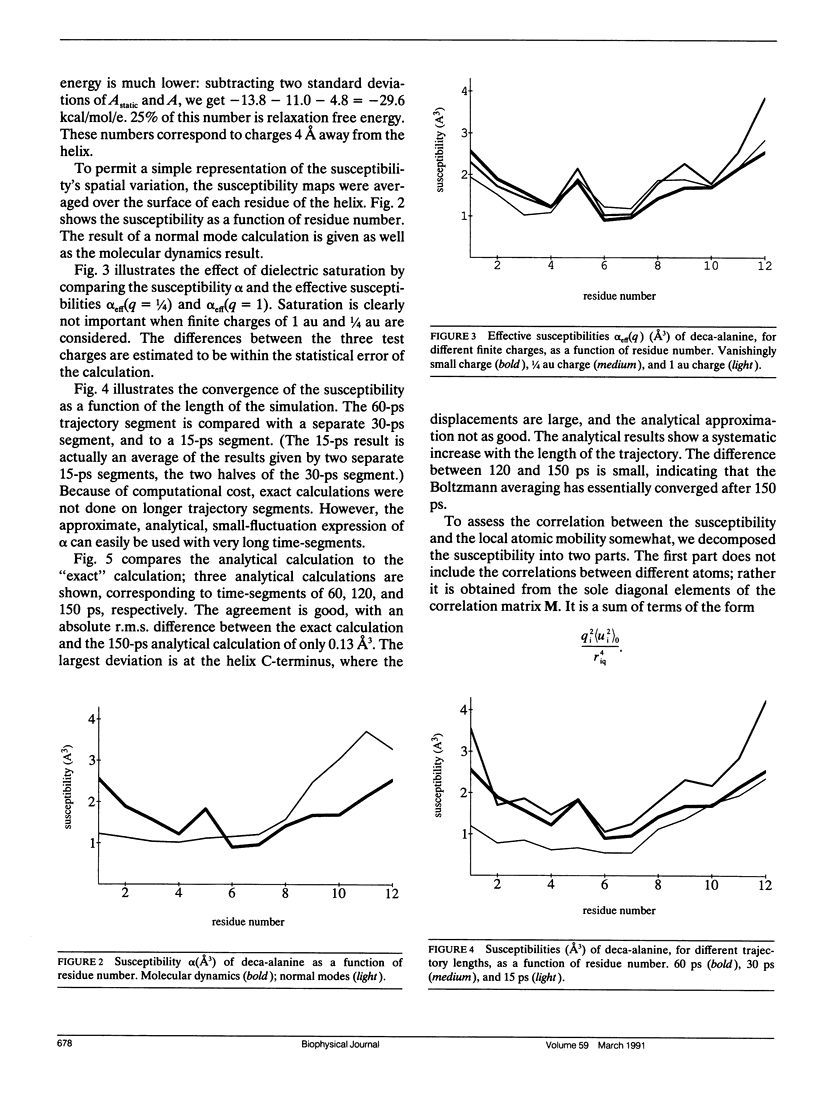
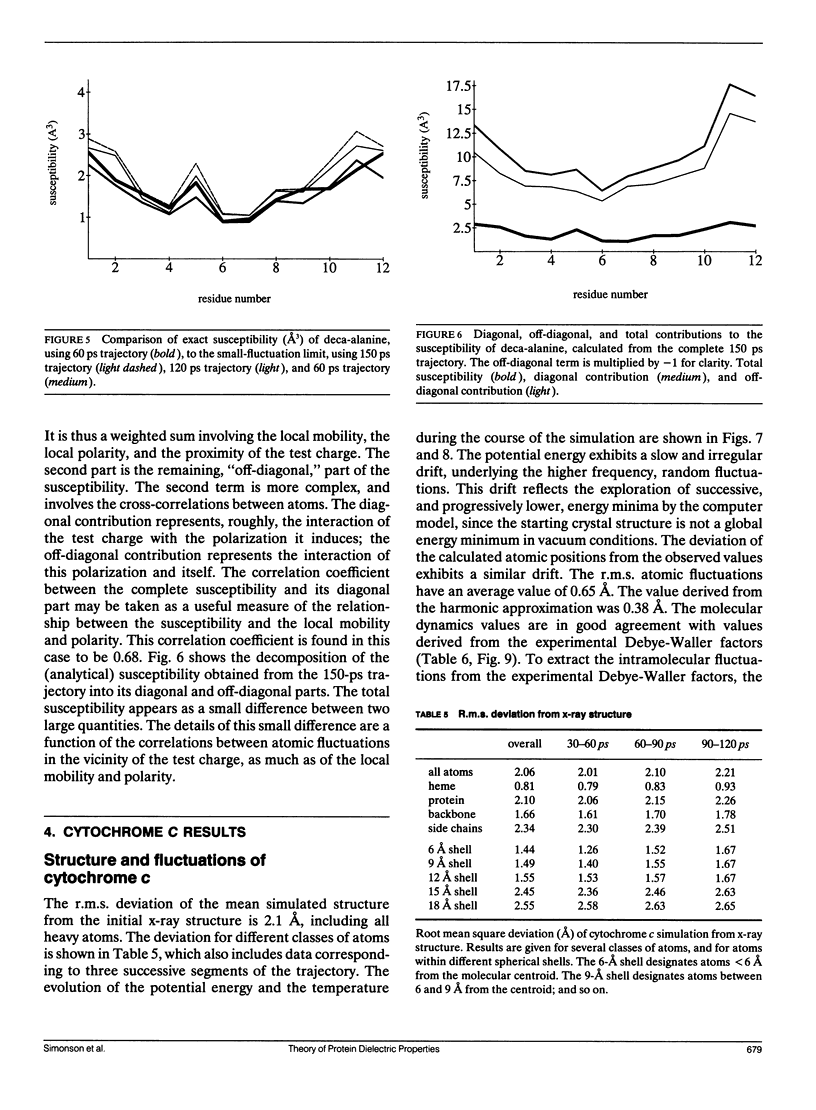
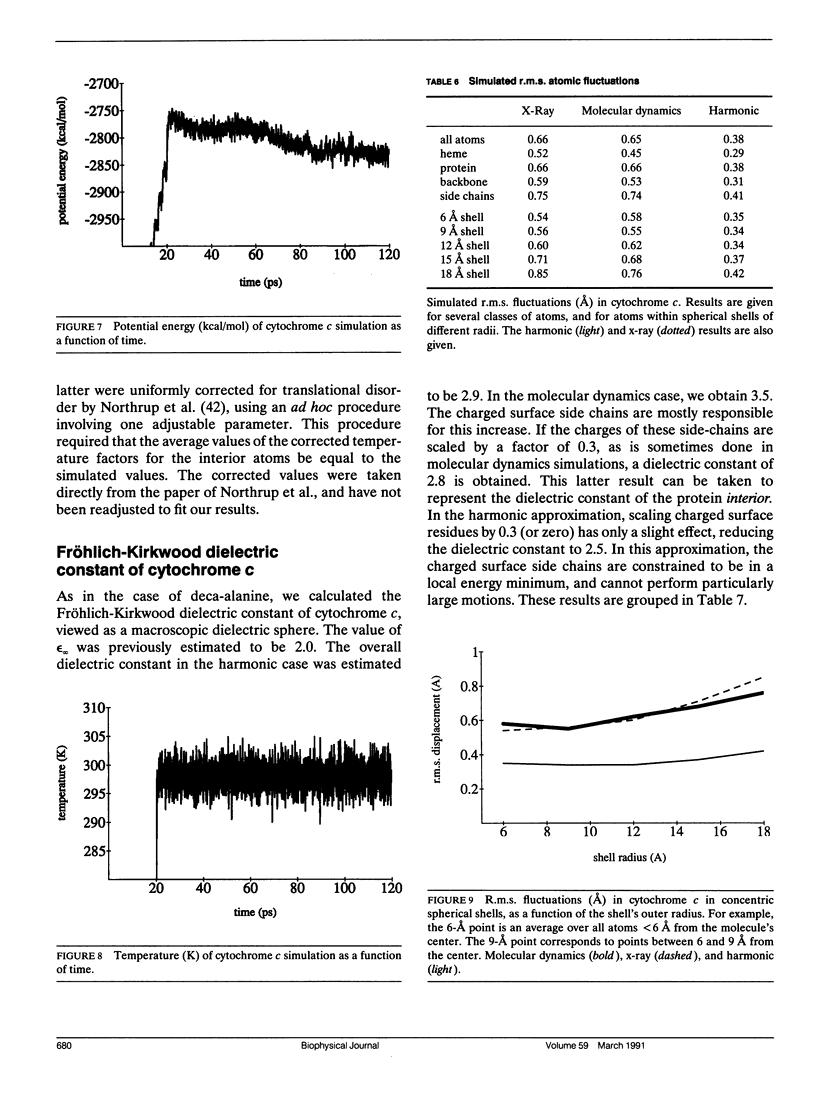
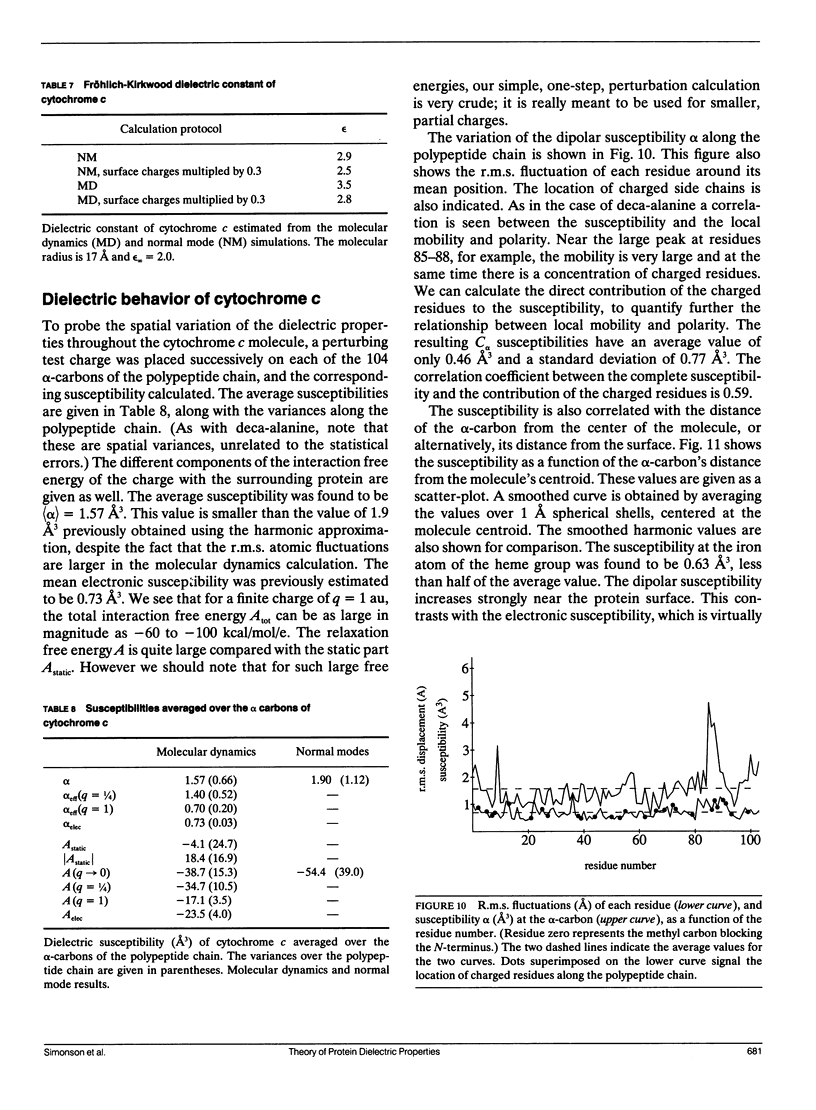
This paper investigates the microscopic mechanisms of charge screening in proteins. The screening of an arbitrary perturbing charge density by a protein and its surrounding solution is characterized by a generalized susceptibility, which is approximately given by the mean dipole-dipole correlation matrix of the system. This susceptibility is a microscopic quantity; the sum of its matrix elements gives the macroscopic susceptibility of continuum electrostatics. When screening of a single perturbing point charge is considered, this susceptibility reduces to a scalar quantity, dependent on position within the protein. The contribution of the positional degrees of freedom of the protein atoms can be estimated from molecular dynamics simulations. This contribution gives rise to large spatial variations of the susceptibility, whose significance for protein function is discussed. The model is applied to the small alpha helix deca-alanine, and to the electron-transfer protein cytochrome c. The results agree qualitatively with previous normal mode calculations. The importance, and the large spatial variations, of charge screening by deca-alanine suggest that dielectric screening may play a role in the binding of charged ligands by helices. In cytochrome c, the dielectric susceptibility in response to a point charge is at a minimum in the central heme region, resulting in a lowering of the reorganization free energy for charge transfer to and from the heme.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone S., Pethig R. Dielectric studies of the binding of water to lysozyme. J Mol Biol. 1982 May 25;157(3):571–575. doi: 10.1016/0022-2836(82)90477-6. [DOI] [PubMed] [Google Scholar]
- Delepierre M., Dobson C. M., Karplus M., Poulsen F. M., States D. J., Wedin R. E. Electrostatic effects and hydrogen exchange behaviour in proteins. The pH dependence of exchange rates in lysozyme. J Mol Biol. 1987 Sep 5;197(1):111–130. doi: 10.1016/0022-2836(87)90613-9. [DOI] [PubMed] [Google Scholar]
- Diamond R. On the use of normal modes in thermal parameter refinement: theory and application to the bovine pancreatic trypsin inhibitor. Acta Crystallogr A. 1990 Jun 1;46(Pt 6):425–435. doi: 10.1107/s0108767390002082. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. The dielectric constant of a folded protein. Biopolymers. 1986 Nov;25(11):2097–2119. doi: 10.1002/bip.360251106. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Hwang J. K., Warshel A. Semiquantitative calculations of catalytic free energies in genetically modified enzymes. Biochemistry. 1987 May 19;26(10):2669–2673. doi: 10.1021/bi00384a003. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Krishtalik L. I. Effective activation energy of enzymatic and nonenzymatic reactions. Evolution-imposed requirements to enzyme structure. J Theor Biol. 1985 Jan 21;112(2):251–264. doi: 10.1016/s0022-5193(85)80285-x. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Perahia D., Karplus M. Molecular dynamics of an alpha-helical polypeptide: Temperature dependence and deviation from harmonic behavior. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1346–1350. doi: 10.1073/pnas.79.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérola F., Rigler R., Holmgren A., Brochon J. C. Picosecond tryptophan fluorescence of thioredoxin: evidence for discrete species in slow exchange. Biochemistry. 1989 Apr 18;28(8):3383–3398. doi: 10.1021/bi00434a038. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Sakamoto T., Wada A. A theoretical study of the dielectric constant of protein. Protein Eng. 1988 Sep;2(3):177–183. doi: 10.1093/protein/2.3.177. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Pear M. R., Morgan J. D., McCammon J. A., Karplus M. Molecular dynamics of ferrocytochrome c. Magnitude and anisotropy of atomic displacements. J Mol Biol. 1981 Dec 25;153(4):1087–1109. doi: 10.1016/0022-2836(81)90469-1. [DOI] [PubMed] [Google Scholar]
- Rao S. N., Singh U. C., Bash P. A., Kollman P. A. Free energy perturbation calculations on binding and catalysis after mutating Asn 155 in subtilisin. Nature. 1987 Aug 6;328(6130):551–554. doi: 10.1038/328551a0. [DOI] [PubMed] [Google Scholar]
- Russell S. T., Warshel A. Calculations of electrostatic energies in proteins. The energetics of ionized groups in bovine pancreatic trypsin inhibitor. J Mol Biol. 1985 Sep 20;185(2):389–404. doi: 10.1016/0022-2836(85)90411-5. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Redox conformation changes in refined tuna cytochrome c. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6371–6375. doi: 10.1073/pnas.77.11.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Schwan H. P. Dielectric dispersion of crystalline powders of amino acids, peptides, and proteins. J Phys Chem. 1965 Dec;69(12):4176–4182. doi: 10.1021/j100782a019. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energetics of enzyme catalysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5250–5254. doi: 10.1073/pnas.75.11.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Warshel A., Naray-Szabo G., Sussman F., Hwang J. K. How do serine proteases really work? Biochemistry. 1989 May 2;28(9):3629–3637. doi: 10.1021/bi00435a001. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T., Churg A. K. Macroscopic models for studies of electrostatic interactions in proteins: limitations and applicability. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4785–4789. doi: 10.1073/pnas.81.15.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Sussman F., Hwang J. K. Evaluation of catalytic free energies in genetically modified proteins. J Mol Biol. 1988 May 5;201(1):139–159. doi: 10.1016/0022-2836(88)90445-7. [DOI] [PubMed] [Google Scholar]
- Warshel A., Sussman F. Toward computer-aided site-directed mutagenesis of enzymes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3806–3810. doi: 10.1073/pnas.83.11.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. What about protein polarity? Nature. 1987 Nov 5;330(6143):15–16. doi: 10.1038/330015a0. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]