Abstract
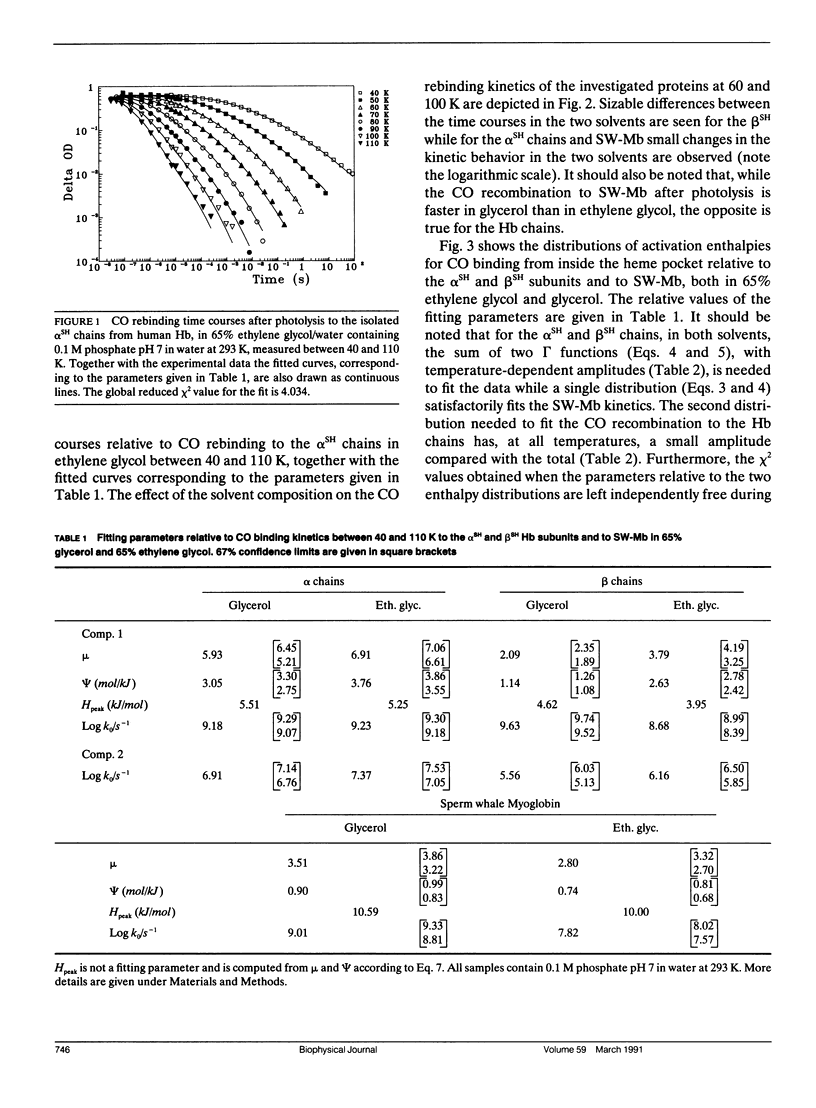
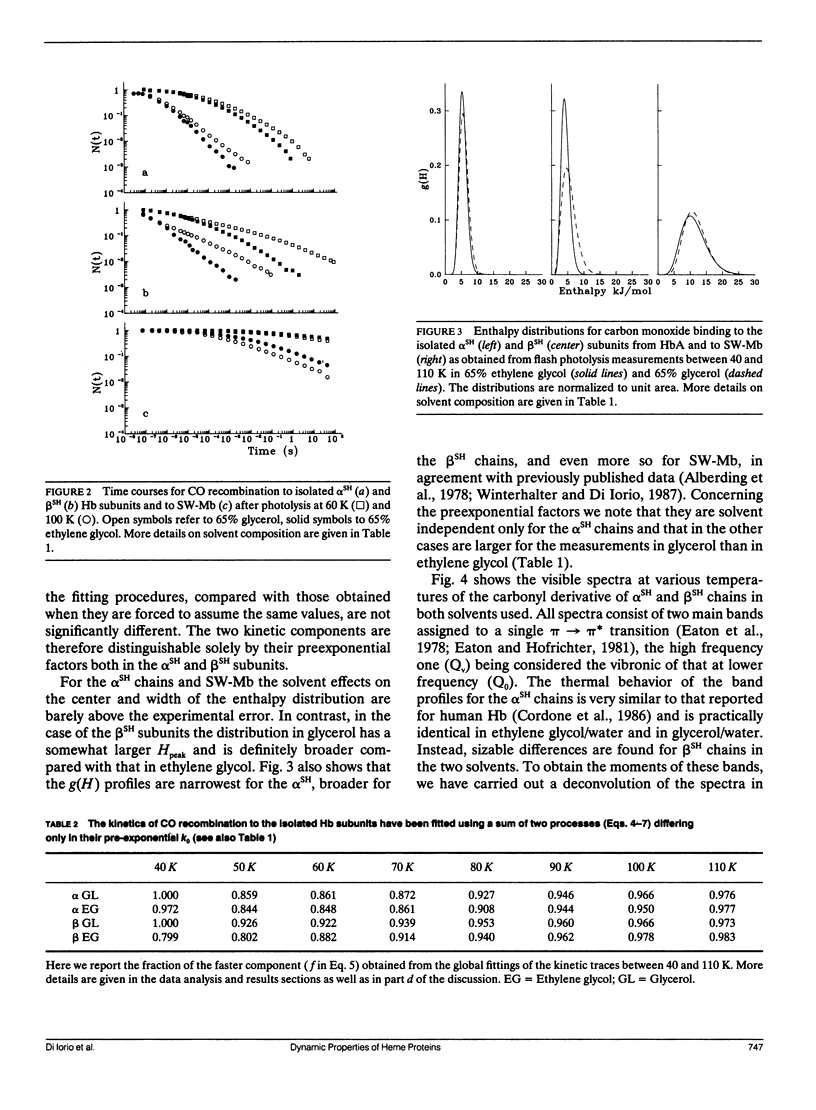
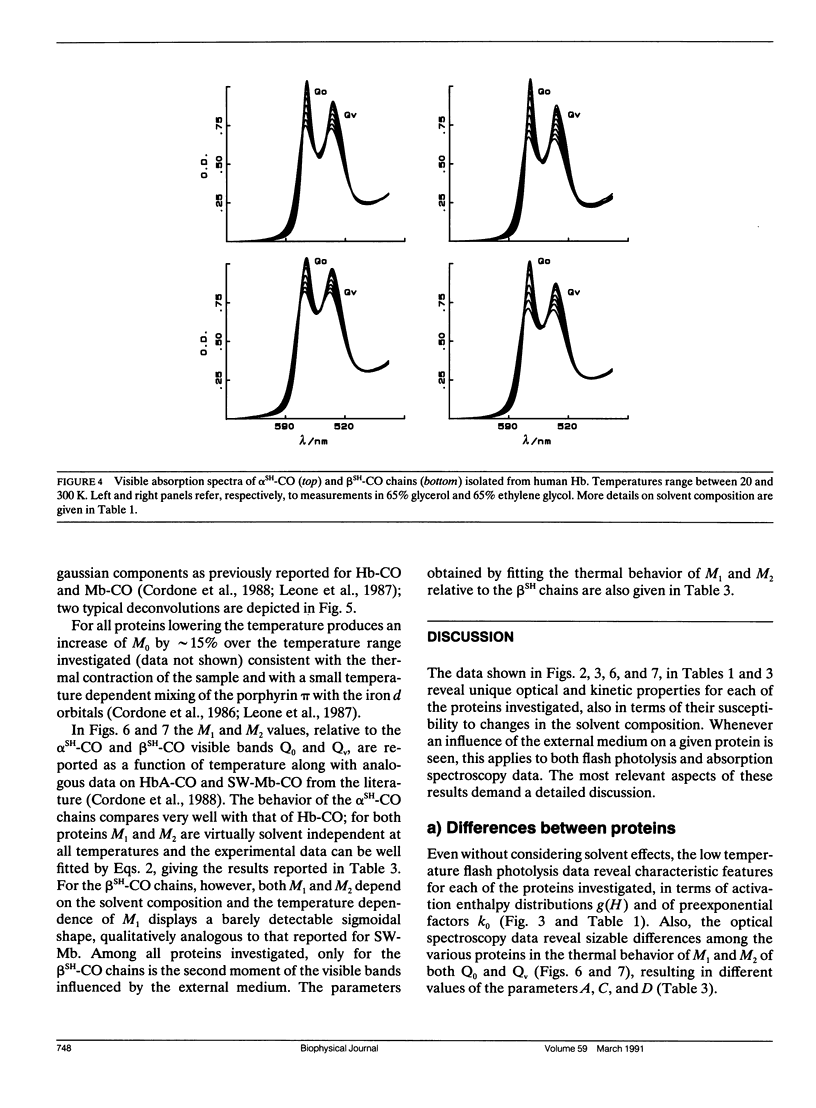
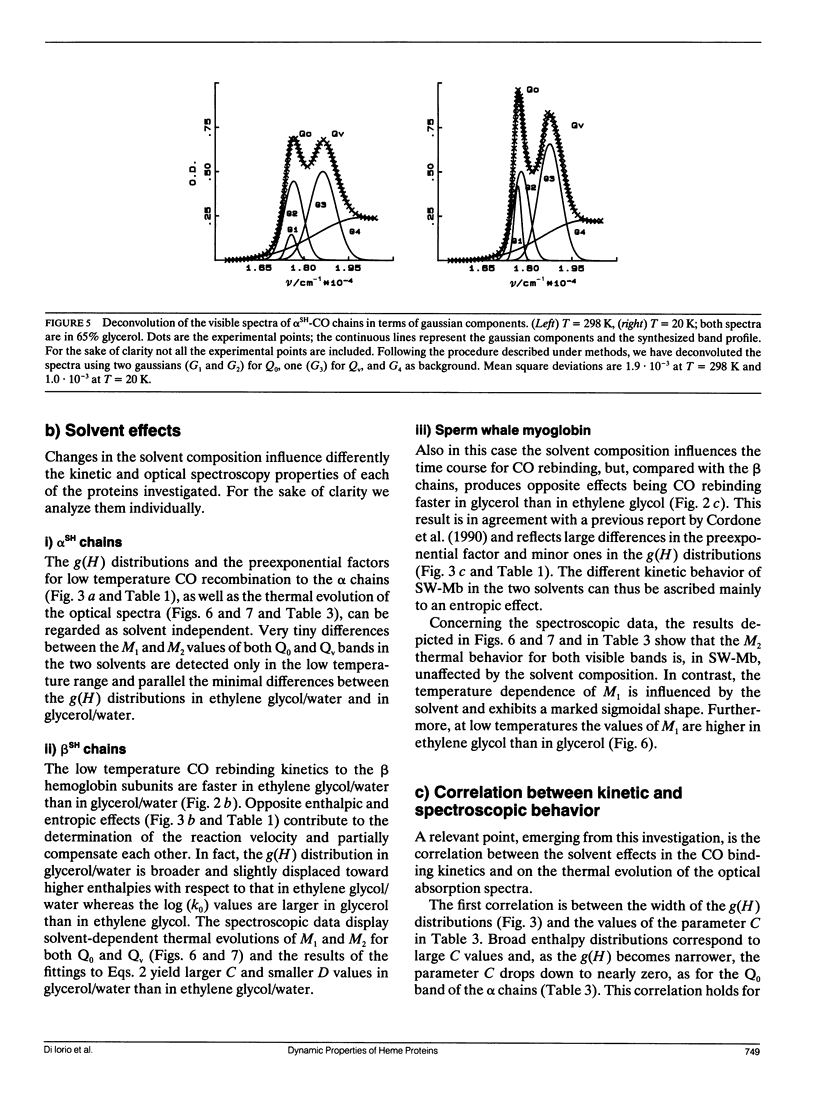
We report the low temperature carbon monoxide recombination kinetics after photolysis and the temperature dependence of the visible absorption spectra of the isolated alpha SH-CO and beta SH-CO subunits from human hemoglobin A in ethylene glycol/water and in glycerol/water mixtures. Kinetic measurements on sperm whale (Physeter catodon) myoglobin and previously published optical spectroscopy data on the latter protein and on human hemoglobin A, in both solvents, (Cordone, L., A. Cupane, M. Leone, E. Vitrano, and D. Bulone. 1988. J. Mol. Biol. 199:312-218) are taken as reference. Low temperature flash photolysis data are analyzed within the multiple substates model proposed by Frauenfelder and co-workers (Austin, R. H., K. W. Beeson, L. Eisenstein, H. Frauenfelder, and I. C. Gunsalus. 1975. Biochemistry. 14:5355-5373). Within this model a distribution of activation enthalpies for ligand binding accounts for the structural heterogeneity of the protein, while the preexponential factor, containing also the entropic contribution to the free energy of the process, is considered to be constant for all conformational substates. Optical spectra are deconvoluted in gaussian components and the temperature dependence of the moments of the resulting bands is analyzed, within the harmonic Frank-Condon approximation, to obtain information on the stereodynamic properties of the heme pocket. The kinetic and spectral parameters thus obtained are found to be protein dependent also with respect to their sensitivity to changes in the composition of the external medium. A close correlation between the kinetic and spectral features is observed for the proteins examined under all experimental conditions studied. The results reported are discussed in terms of differences in the heme pocket structure and in the conformational heterogeneity among the various proteins, as related to their different capability to accommodate constraints imposed by the external medium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Ansari A., DiIorio E. E., Dlott D. D., Frauenfelder H., Iben I. E., Langer P., Roder H., Sauke T. B., Shyamsunder E. Ligand binding to heme proteins: relevance of low-temperature data. Biochemistry. 1986 Jun 3;25(11):3139–3146. doi: 10.1021/bi00359a011. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Braunstein D., Ansari A., Berendzen J., Cowen B. R., Egeberg K. D., Frauenfelder H., Hong M. K., Ormos P., Sauke T. B., Scholl R. Ligand binding to synthetic mutant myoglobin (His-E7----Gly): role of the distal histidine. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8497–8501. doi: 10.1073/pnas.85.22.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci E. Preparation of isolated chains of human hemoglobin. Methods Enzymol. 1981;76:97–106. doi: 10.1016/0076-6879(81)76117-2. [DOI] [PubMed] [Google Scholar]
- Congiu Castellano A., Castagnola M., Burattini E., Dell'Ariccia M., Della Longa S., Giovannelli A., Durham P. J., Bianconi A. Heterogeneity of the isolated subunits of the fetal and adult human hemoglobin in solution, detected by XANES spectroscopy. Biochim Biophys Acta. 1989 Jul 6;996(3):240–246. doi: 10.1016/0167-4838(89)90253-7. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E., Bulone D. Interaction between external medium and haem pocket in myoglobin probed by low-temperature optical spectroscopy. J Mol Biol. 1988 Jan 5;199(1):213–218. doi: 10.1016/0022-2836(88)90390-7. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys Chem. 1986 Aug;24(3):259–275. doi: 10.1016/0301-4622(86)85031-1. [DOI] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Thermal behavior of the 760-nm absorption band in photodissociated sperm whale carbonmonoxymyoglobin at cryogenic temperature: dependence on external medium. Biopolymers. 1990 Feb 15;29(3):639–643. doi: 10.1002/bip.360290316. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Structural and dynamic properties of the heme pocket in myoglobin probed by optical spectroscopy. Biopolymers. 1988 Dec;27(12):1977–1997. doi: 10.1002/bip.360271209. [DOI] [PubMed] [Google Scholar]
- Dlott D. D., Frauenfelder H., Langer P., Roder H., DiIorio E. E. Nanosecond flash photolysis study of carbon monoxide binding to the beta chain of hemoglobin Zürich [beta 63(E7)His leads to Arg]. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6239–6243. doi: 10.1073/pnas.80.20.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Wolynes P. G. Rate theories and puzzles of hemeprotein kinetics. Science. 1985 Jul 26;229(4711):337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Ikeda-Saito M., Inubushi T., Yonetani T. Preparation of hybrid hemoglobins with different prosthetic groups. Methods Enzymol. 1981;76:113–121. doi: 10.1016/0076-6879(81)76119-6. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Leone M., Cupane A., Vitrano E., Cordone L. Dynamic properties of oxy- and carbonmonoxyhemoglobin probed by optical spectroscopy in the temperature range of 300-20 K. Biopolymers. 1987 Oct;26(10):1769–1779. doi: 10.1002/bip.360261009. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Colosimo A. Chromatographic isolation and characterization of isolated chains from hemoglobin after regeneration of sulfhydryl groups. Biochemistry. 1971 Feb 16;10(4):621–624. doi: 10.1021/bi00780a012. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Di Iorio E. E. Deformability of the hemoglobin molecule as the basis of its functional behavior. Acta Haematol. 1987;78(2-3):90–94. doi: 10.1159/000205852. [DOI] [PubMed] [Google Scholar]


