Abstract
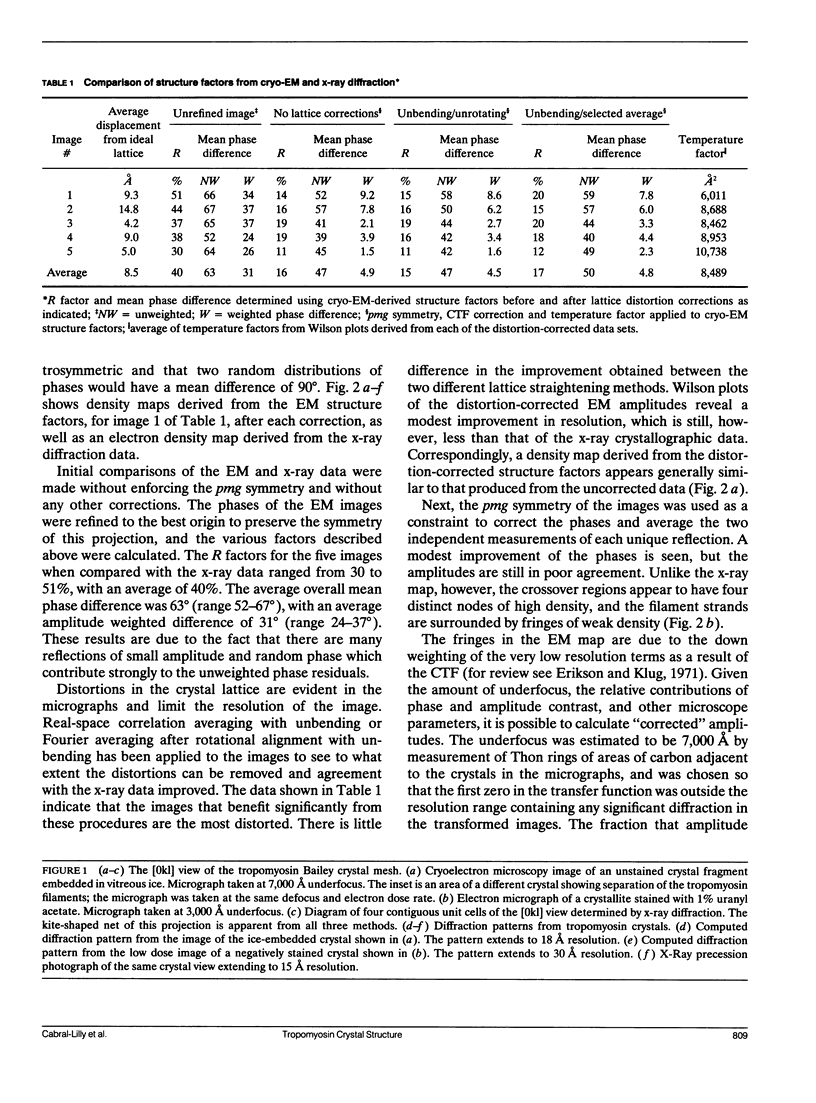
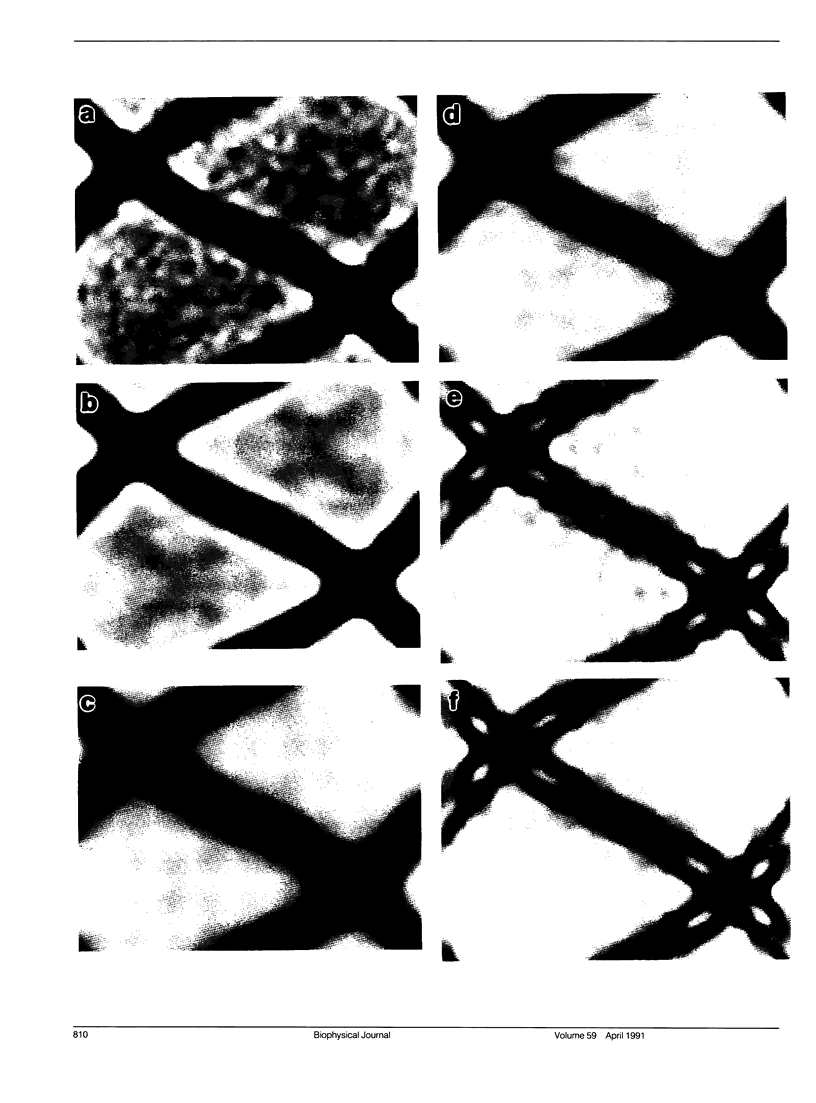
A comparison has been made between cryoelectron microscope images and the x-ray structure of one projection of the Bailey tropomyosin crystal. The computed transforms of the electron micrographs extend to a resolution of approximately 18 A compared with the reflections from x-ray crystallography which extend to 15 A. After correction of the images for lattice distortions and the contrast transfer function, the structure factors were constrained to the plane group (pmg) symmetry of this projection. Amplitude and phase data for five images were compared with the corresponding view from the three-dimensional x-ray diffraction data (Phillips, G.N., Jr., J.P. Fillers, and C. Cohen. 1986. J. Mol. Biol. 192: 111-131). The average R factor between the electron microscopy and x-ray amplitudes was 15%, with an amplitude-weighted mean phase difference of 4.8 degrees. The density maps derived from cryoelectron microscopy contain structural features similar to those from x-ray diffraction: these include the width and run of the filaments and their woven appearance at the crossover regions. Preliminary images obtained from frozen-hydrated tropomyosin/troponin cocrystals suggest that this approach may provide structural details not readily obtainable from x-ray diffraction studies.
Full text
PDF


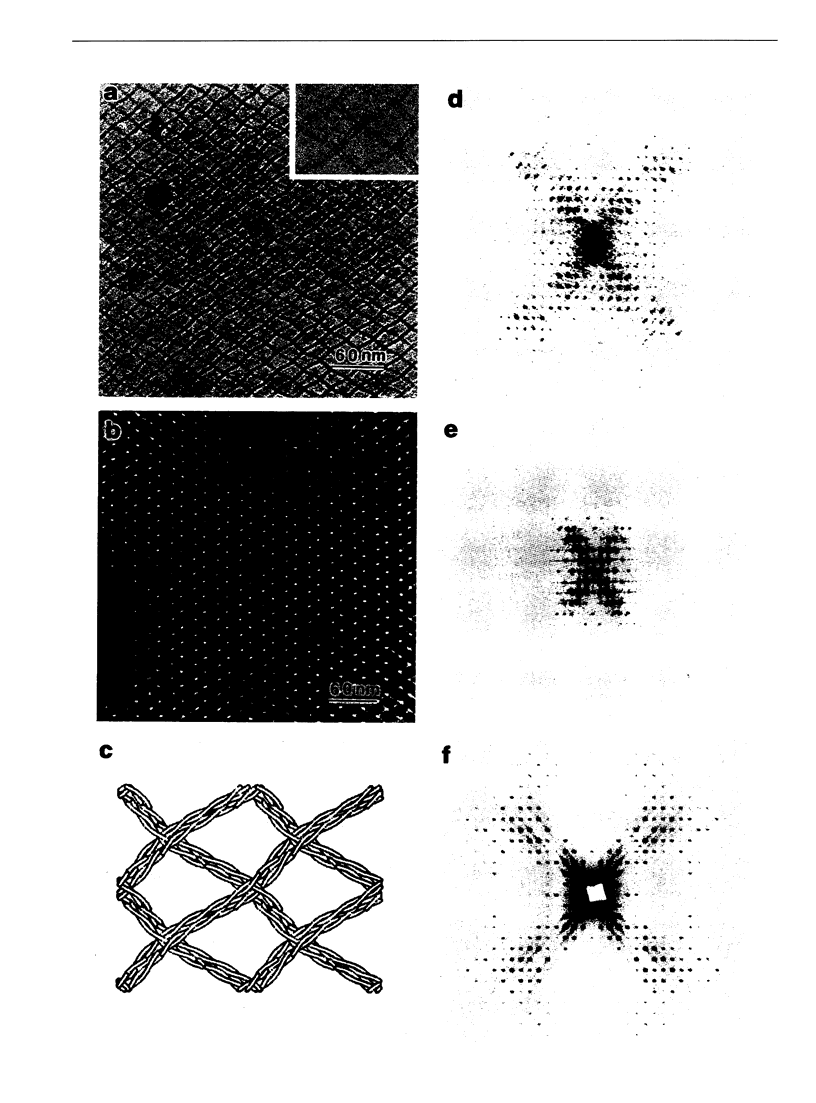


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Carr H. J., O'Brien E. J., Morris E. P. Structure of tropomyosin-troponin T cocrystals. J Muscle Res Cell Motil. 1988 Oct;9(5):384–392. doi: 10.1007/BF01774065. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Cohen C., Longley W. Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol. 1969 Apr 14;41(1):87–107. doi: 10.1016/0022-2836(69)90128-4. [DOI] [PubMed] [Google Scholar]
- Chiu W. Electron microscopy of frozen, hydrated biological specimens. Annu Rev Biophys Biophys Chem. 1986;15:237–257. doi: 10.1146/annurev.bb.15.060186.001321. [DOI] [PubMed] [Google Scholar]
- Cohen C., Caspar D. L., Parry D. A., Lucas R. M. Tropomyosin crystal dynamics. Cold Spring Harb Symp Quant Biol. 1972;36:205–216. doi: 10.1101/sqb.1972.036.01.028. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Greaser M. L., Yamaguchi M., Vanderkooi G. Crystal forms of alpha2-tropomyosin. J Mol Biol. 1977 Nov;116(4):883–890. doi: 10.1016/0022-2836(77)90277-7. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Higashi S., Ooi T. Crystals of tropomyosin and native tropomyosin. J Mol Biol. 1968 Jun 28;34(3):699–701. doi: 10.1016/0022-2836(68)90190-3. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Downing K. H., Walian P. J. Structure of PhoE porin in projection at 3.5 A resolution. J Struct Biol. 1990 Mar;103(1):57–63. doi: 10.1016/1047-8477(90)90086-r. [DOI] [PubMed] [Google Scholar]
- Jeng T. W., Crowther R. A., Stubbs G., Chiu W. Visualization of alpha-helices in tobacco mosaic virus by cryo-electron microscopy. J Mol Biol. 1989 Jan 5;205(1):251–257. doi: 10.1016/0022-2836(89)90379-3. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Cohen C., Stewart M. A new crystal form of tropomyosin. Preliminary X-ray diffraction analysis. J Mol Biol. 1987 May 5;195(1):219–223. doi: 10.1016/0022-2836(87)90339-1. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Motions of tropomyosin. Crystal as metaphor. Biophys J. 1980 Oct;32(1):485–502. doi: 10.1016/S0006-3495(80)84985-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., DeRosier D. A surveying optical diffractometer. J Microsc. 1981 Sep;123(Pt 3):239–247. doi: 10.1111/j.1365-2818.1981.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Sosinsky G. E., Baker T. S., Caspar D. L., Goodenough D. A. Correlation analysis of gap junction lattice images. Biophys J. 1990 Nov;58(5):1213–1226. doi: 10.1016/S0006-3495(90)82462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. Crystalline sheets of tropomyosin. J Mol Biol. 1984 Mar 25;174(1):231–238. doi: 10.1016/0022-2836(84)90375-9. [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Contrast transfer for frozen-hydrated specimens: determination from pairs of defocused images. Ultramicroscopy. 1988;25(4):279–291. doi: 10.1016/0304-3991(88)90003-4. [DOI] [PubMed] [Google Scholar]
- White S. P., Cohen C., Phillips G. N., Jr Structure of co-crystals of tropomyosin and troponin. 1987 Feb 26-Mar 4Nature. 325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]