Abstract
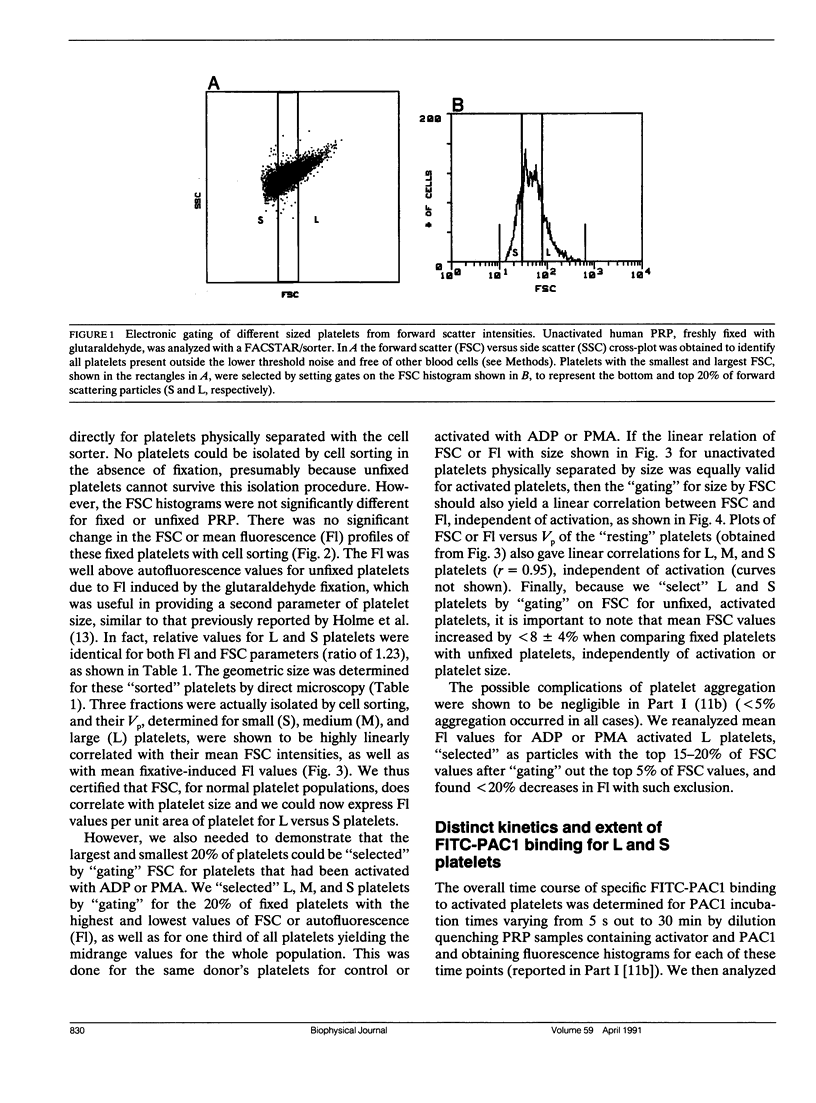
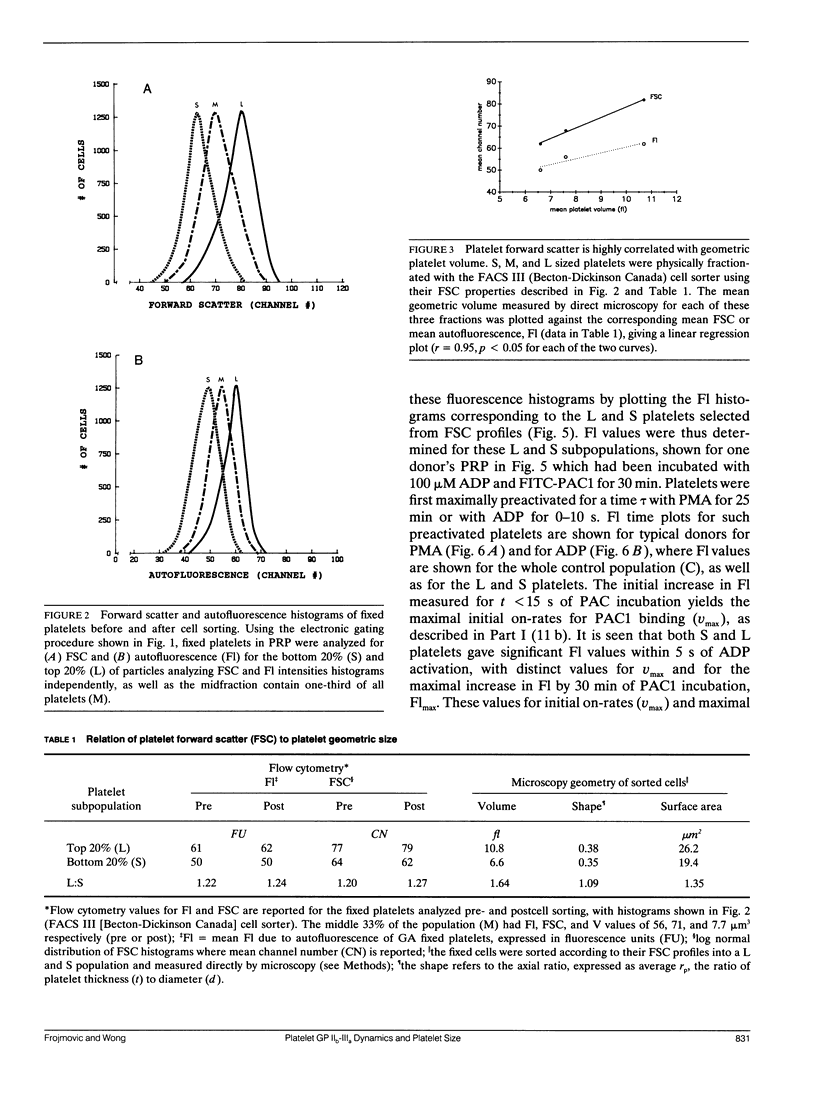
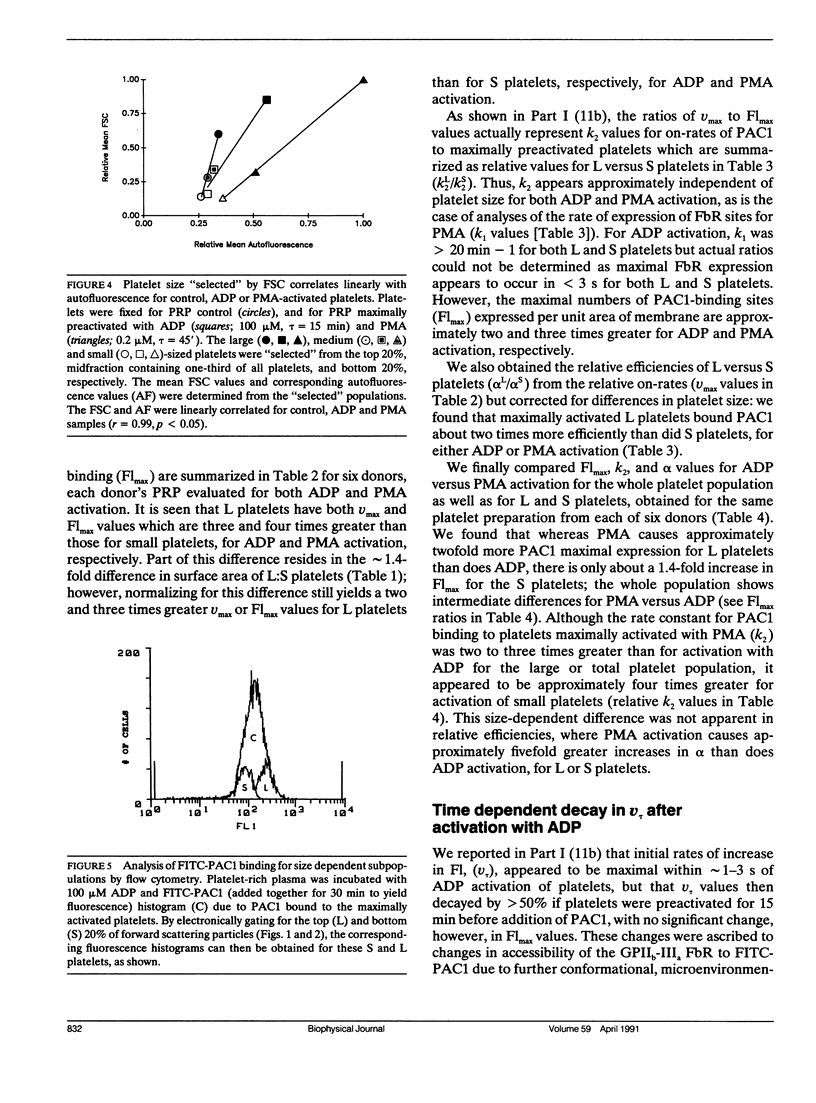
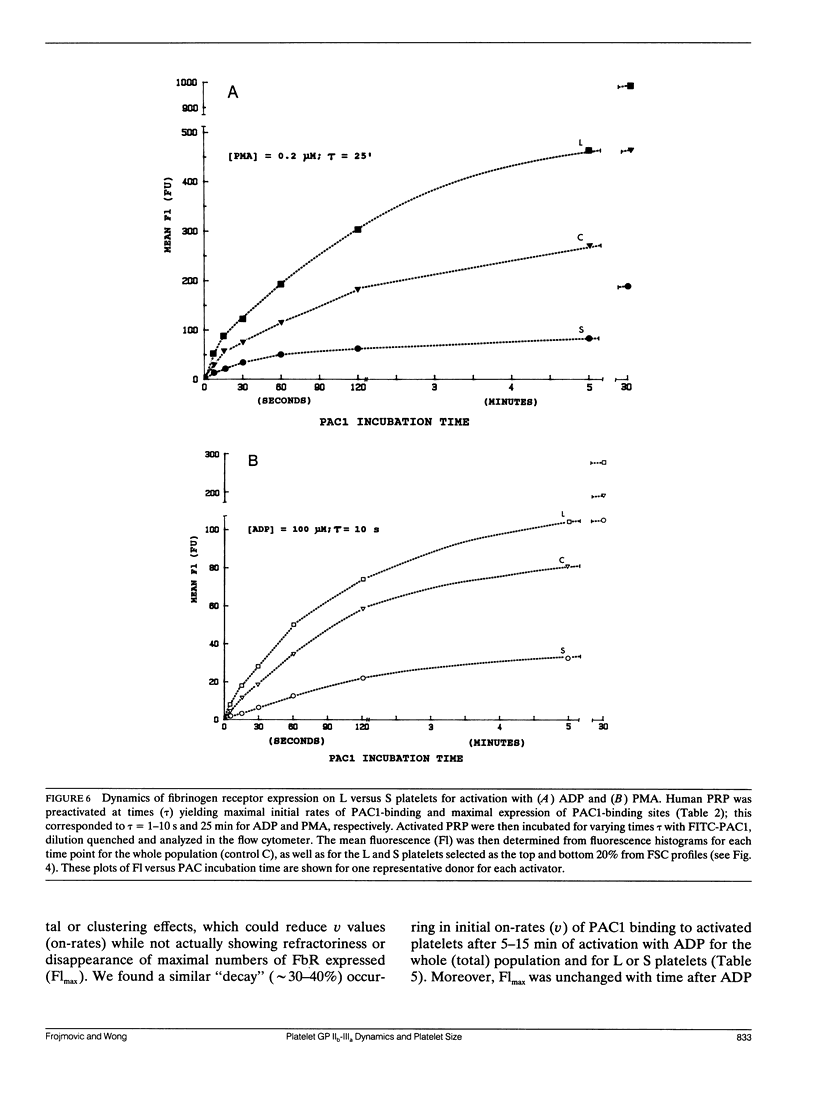
Platelet aggregation has previously been shown to occur within 1 s of activation with 100 microM adenosine diphosphate (ADP) for both large (L) and small (S) platelet subpopulations, but L platelets were about twofold more sensitive and more rapidly recruited into microaggregates than were S platelets after correcting for differences in platelet surface area. Because platelet aggregation normally requires fibrinogen binding to glycoprotein IIb-IIIa receptors (FbR) expressed on the activated platelet surface, we wished to compare the kinetics and nature of FbR expression induced by ADP for L versus S platelets, and to measure size-dependent differences in FbR expression for platelets maximally activated with phorbol myristate acetate (PMA). We presented the theory and methodology in Part I (Frojmovic, M., T. Wong, and T. van de Ven. 1991. Biophys. J. 59:815-827) for measuring the rate of FbR expression (k1) and both the rate (k2) and efficiency (alpha) of binding of PAC1 to FbR as a function of activation conditions from the initial on-rate of FITC-PAC1 to FbR (V) and the maximal number of FbR expressed: these are measured, respectively, from the initial rate of increase in platelet-bound fluorescence (v) and the maximal increase in mean fluorescence (Flmax). We extended these analyses to L and S platelets, selected by electronic gating of forward scatter profiles (FSC), with corresponding fluorescence (Fl) histograms retrieved analytically. Platelet size (V) and surface area (SA), determined directly for cells separated with a cell sorter, were highly correlated with FSC, allowing v and Flmax values to be expressed per unit area of membrane for L:S comparisons. Surprisingly, ADP activation appeared to express all FbR within 1-3 s of ADP activation for both L and S platelets, whereas k1 was similar for PMA activation. In addition, L platelets maximally expressed two and three times more FbR per unit area than did S platelets when maximally stimulated, respectively, with ADP or PMA. Whereas k2 was independent of platelet size for a given activator, the efficiency of PAC1 binding (alpha), per unit area of membrane, was two times greater for L than for S platelets, for either ADP or PMA activation. Our data suggest that the FbR structure, its microenvironment, or its surface organization may vary with platelet size or activator type. Major reorganization of FbR and/or its environment appears to occur after approximately 5 min of ADP activation equally for both L and S platelets. A model is presented to account for size-dependent differences in FbR expression with implications for regulation of platelet aggregation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carty D. J., Gear A. R. Fractionation of platelets according to size: functional and biochemical characteristics. Am J Hematol. 1986 Jan;21(1):1–14. doi: 10.1002/ajh.2830210102. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M. Comparative studies of turbidimetrically measured rates of platelet aggregation require adjustment of the platelet suspension according to the mean relative size and optical efficiency of the platelets used. Blood. 1989 Nov 1;74(6):2302–2303. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G., Duchastel A. Microscopic measurements of platelet aggregation reveal a low ADP-dependent process distinct from turbidometrically measured aggregation. J Lab Clin Med. 1983 Jun;101(6):964–976. [PubMed] [Google Scholar]
- Frojmovic M. M., Milton J. G. Human platelet size, shape, and related functions in health and disease. Physiol Rev. 1982 Jan;62(1):185–261. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Panjwani R. Geometry of normal mammalian platelets by quantitative microscopic studies. Biophys J. 1976 Sep;16(9):1071–1089. doi: 10.1016/S0006-3495(76)85756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojmovic M., Wong T., van de Ven T. Dynamic measurements of the platelet membrane glycoprotein IIb-IIIa receptor for fibrinogen by flow cytometry. I. Methodology, theory and results for two distinct activators. Biophys J. 1991 Apr;59(4):815–827. doi: 10.1016/S0006-3495(91)82294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haver V. M., Gear A. R. Functional fractionation of platelets: aggregation kinetics and glycoprotein labeling of differing platelet population. Thromb Haemost. 1982 Oct 29;48(2):211–216. [PubMed] [Google Scholar]
- Holme S., Heaton A., Konchuba A., Hartman P. Light scatter and total protein signal distribution of platelets by flow cytometry as parameters of size. J Lab Clin Med. 1988 Aug;112(2):223–231. [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978 Feb;51(2):307–316. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McGann L. E., Walterson M. L., Hogg L. M. Light scattering and cell volumes in osmotically stressed and frozen-thawed cells. Cytometry. 1988 Jan;9(1):33–38. doi: 10.1002/cyto.990090106. [DOI] [PubMed] [Google Scholar]
- Milton J. G. Dependence of platelet volume measurements on heterogeneity of platelet morphology. Biophys J. 1981 Jul;35(1):257–261. doi: 10.1016/S0006-3495(81)84787-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington D. G., Streatfield K., Roxburgh A. E. Megakaryocytes and the heterogeneity of circulating platelets. Br J Haematol. 1976 Dec;34(4):639–653. doi: 10.1111/j.1365-2141.1976.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Thompson C. B., Eaton K. A., Princiotta S. M., Rushin C. A., Valeri C. R. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982 Mar;50(3):509–519. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Jakubowski J. A., Quinn P. G., Deykin D., Valeri C. R. Platelet size as a determinant of platelet function. J Lab Clin Med. 1983 Feb;101(2):205–213. [PubMed] [Google Scholar]
- Thompson C. B. Selective consumption of large platelets during massive bleeding. Br Med J (Clin Res Ed) 1985 Jul 13;291(6488):95–96. doi: 10.1136/bmj.291.6488.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencel-Drake J. D., Plow E. F., Kunicki T. J., Woods V. L., Keller D. M., Ginsberg M. H. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol. 1986 Aug;124(2):324–334. [PMC free article] [PubMed] [Google Scholar]
- Wong T., Pedvis L., Frojmovic M. Platelet size affects both micro- and macro-aggregation: contributions of platelet number, volume fraction and cell surface. Thromb Haemost. 1989 Sep 29;62(2):733–741. [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]


