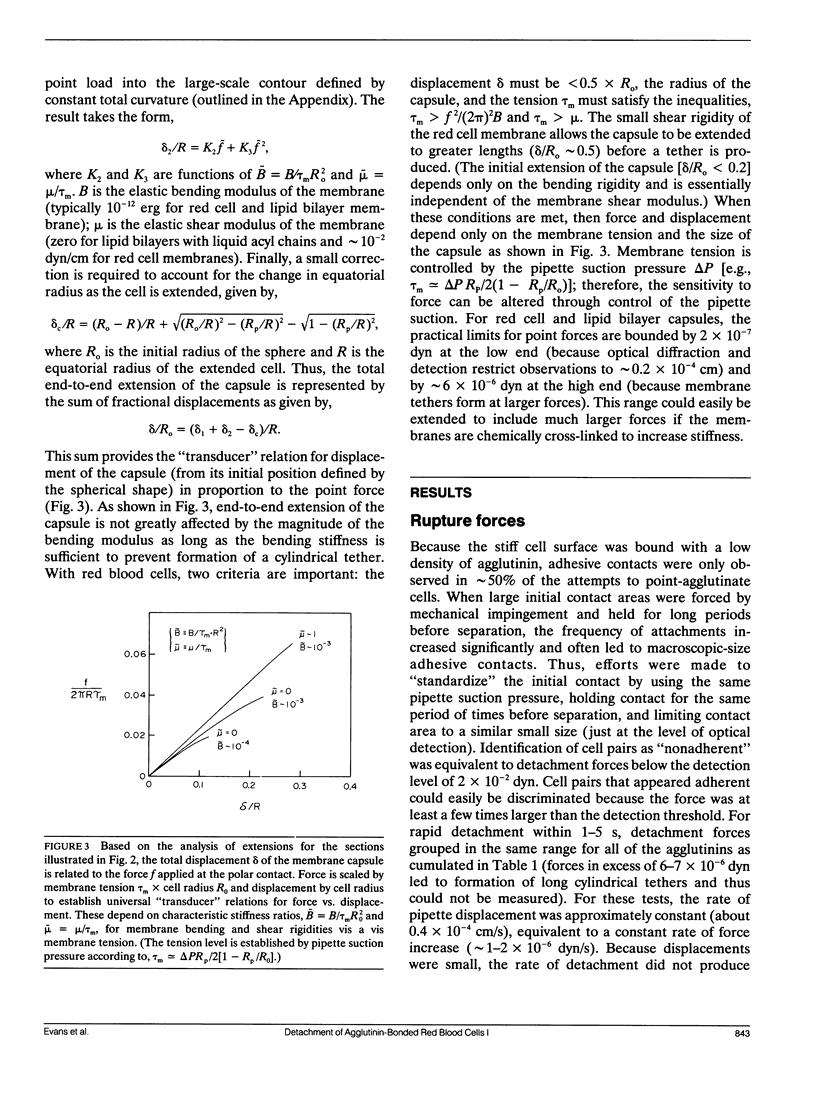
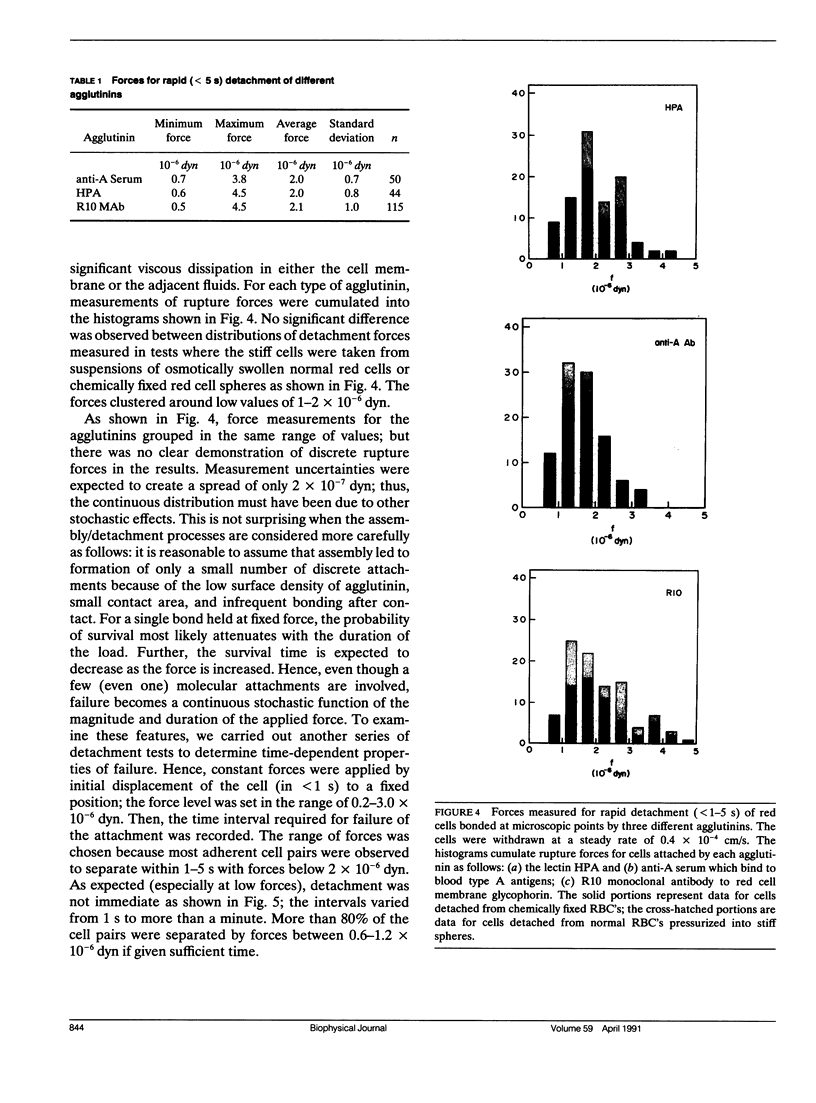
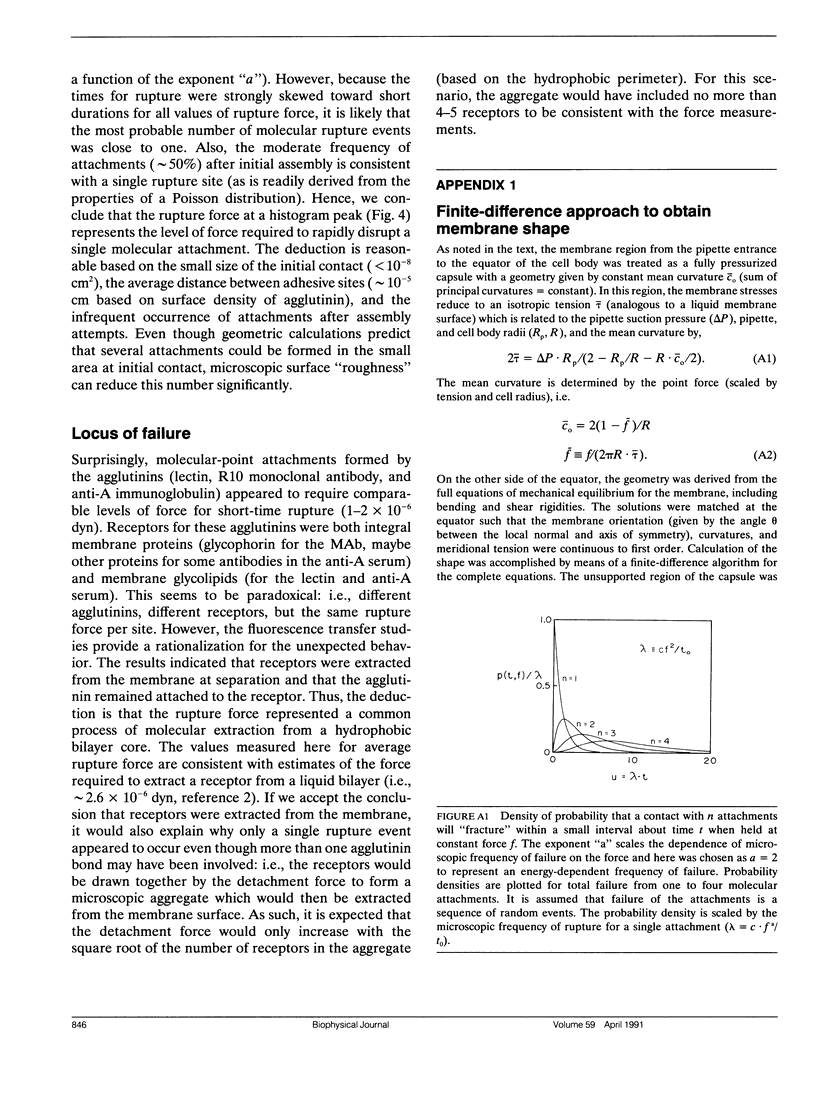
Abstract
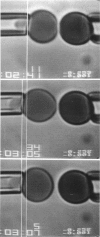
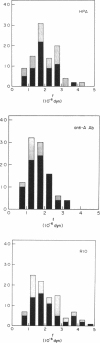
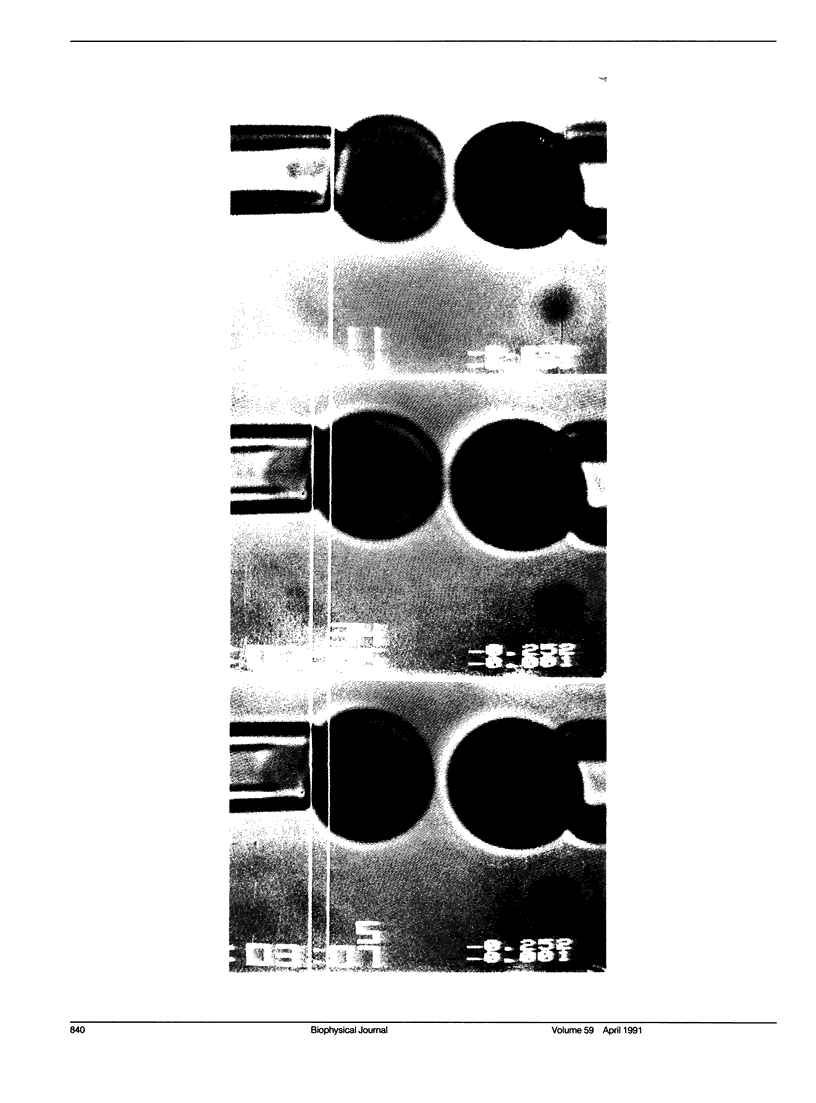
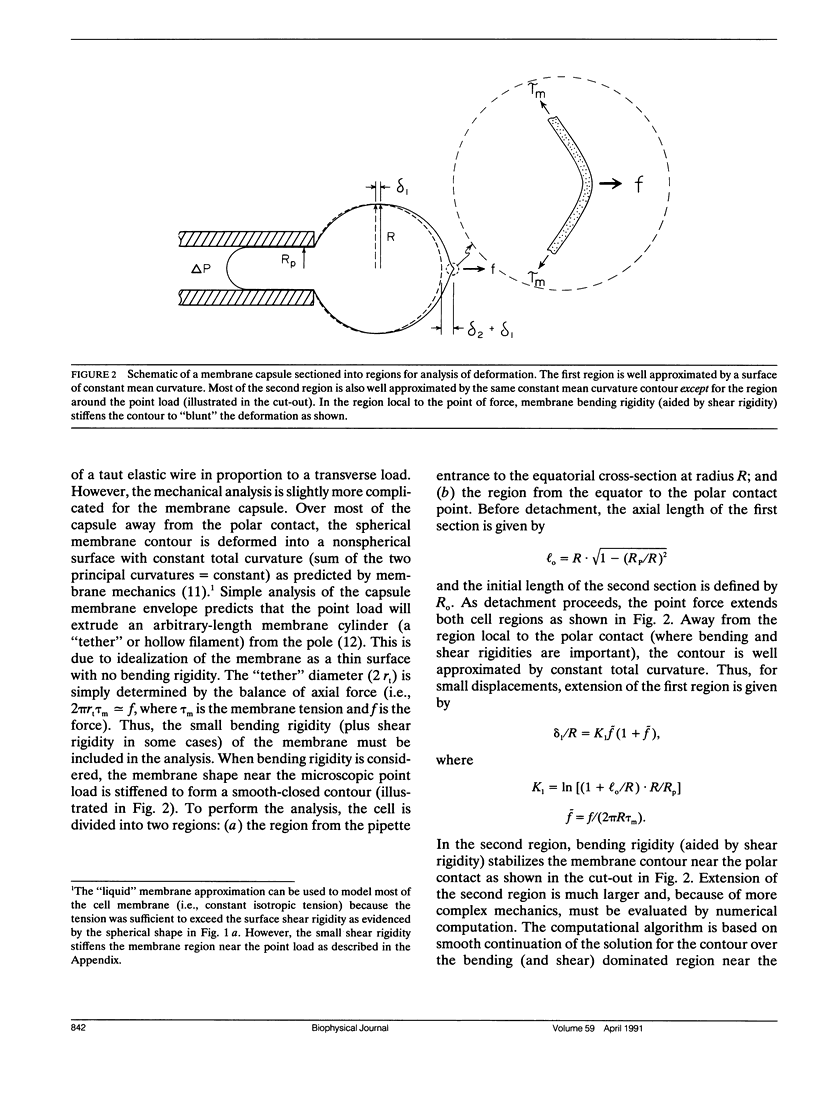
A simple micromechanical method has been developed to measure the rupture strength of a molecular-point attachment (focal bond) between two macroscopically smooth membrane capsules. In the procedure, one capsule is prepared with a low density coverage of adhesion molecules, formed as a stiff sphere, and held at fixed position by a micropipette. The second capsule without adhesion molecules is pressurized into a spherical shape with low suction by another pipette. This capsule is maneuvered to initiate point contact at the pole opposite the stiff capsule which leads to formation of a few (or even one) molecular attachments. Then, the deformable capsule is slowly withdrawn by displacement of the pipette. Analysis shows that the end-to-end extension of the capsule provides a direct measure of the force at the point contact and, therefore, the rupture strength when detachment occurs. The range for point forces accessible to this technique depends on the elastic moduli of the membrane, membrane tension, and the size of the capsule. For biological and synthetic vesicle membranes, the range of force lies between 10(-7)-10(-5) dyn (10(-12)-10(-10) N) which is 100-fold less than presently measurable by Atomic Force Microscopy! Here, the approach was used to study the forces required to rupture microscopic attachments between red blood cells formed by a monoclonal antibody to red cell membrane glycophorin, anti-A serum, and a lectin from the snail-helix pomatia. Failure of the attachments appeared to be a stochastic function of the magnitude and duration of the detachment force. We have correlated the statistical behavior observed for rupture with a random process model for failure of small numbers of molecular attachments. The surprising outcome of the measurements and analysis was that the forces deduced for short-time failure of 1-2 molecular attachments were nearly the same for all of the agglutinin, i.e., 1-2 x 10(-6) dyn. Hence, microfluorometric tests were carried out to determine if labeled agglutinins and/or labeled surface molecules were transferred between surfaces after separation of large areas of adhesive contact. The results showed that the attachments failed because receptors were extracted from the membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Evans E., Berk D., Leung A., Mohandas N. Detachment of agglutinin-bonded red blood cells. II. Mechanical energies to separate large contact areas. Biophys J. 1991 Apr;59(4):849–860. doi: 10.1016/S0006-3495(91)82297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Kabat E. A. Studies on specificity and binding properties of the blood group A reactive hemagglutinin from Helix pomatia. Biochemistry. 1971 Apr 27;10(9):1684–1692. doi: 10.1021/bi00785a028. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Evans E. A. Extensional flow of erythrocyte membrane from cell body to elastic tether. I. Analysis. Biophys J. 1982 Jul;39(1):71–81. doi: 10.1016/S0006-3495(82)84492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Wrb antigen, a receptor for Plasmodium falciparum malaria, is located on a helical region of the major membrane sialoglycoprotein of human red blood cells. Biochem J. 1983 Jan 1;209(1):273–276. doi: 10.1042/bj2090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha S. P., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. I. Theoretical. Biophys J. 1986 Dec;50(6):1109–1116. doi: 10.1016/S0006-3495(86)83555-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha S. P., Shuster J., Goldsmith H. L. Interaction forces between red cells agglutinated by antibody. II. Measurement of hydrodynamic force of breakup. Biophys J. 1986 Dec;50(6):1117–1126. doi: 10.1016/S0006-3495(86)83556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]