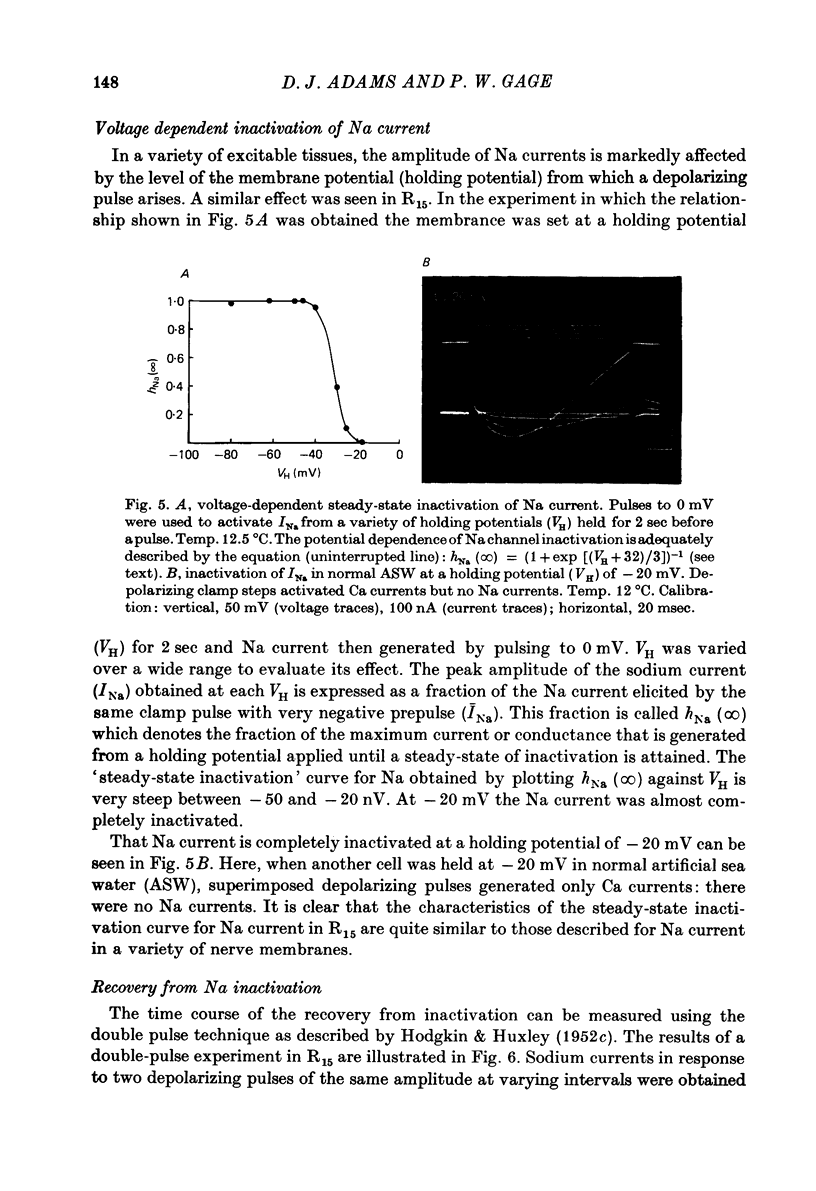
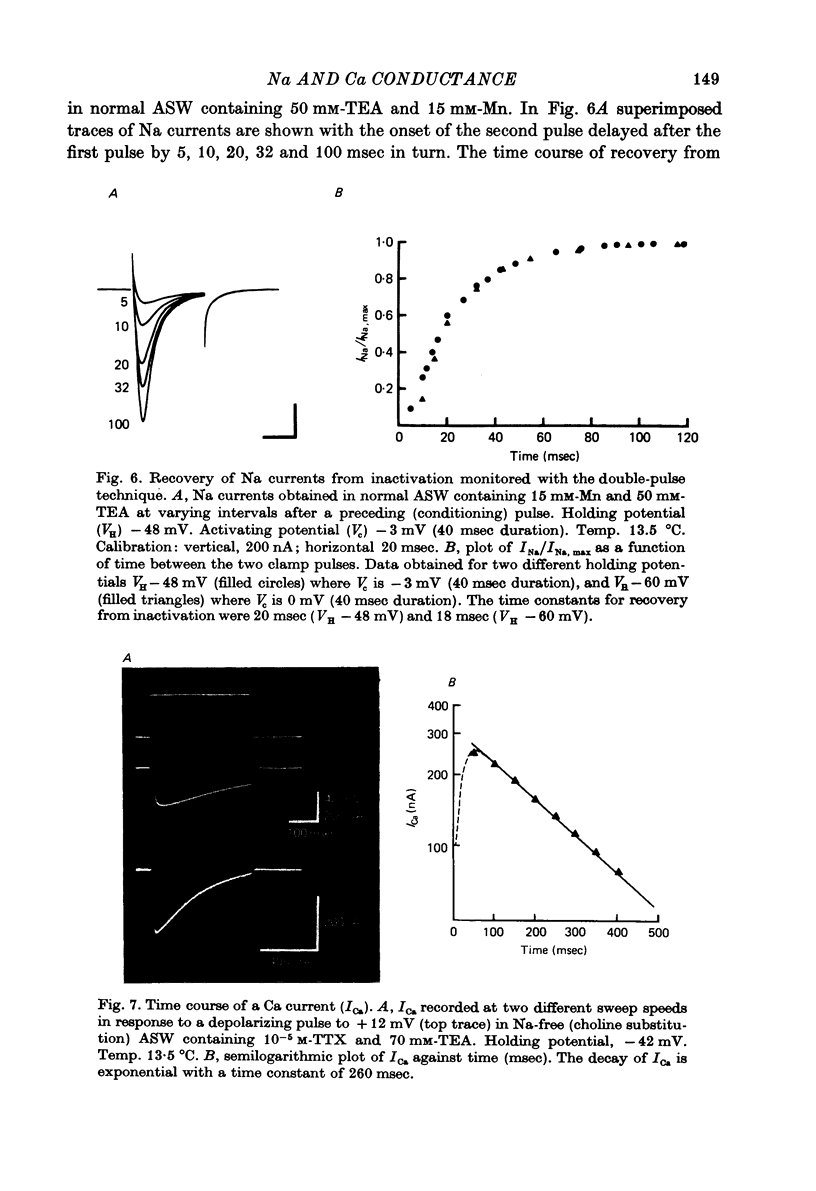
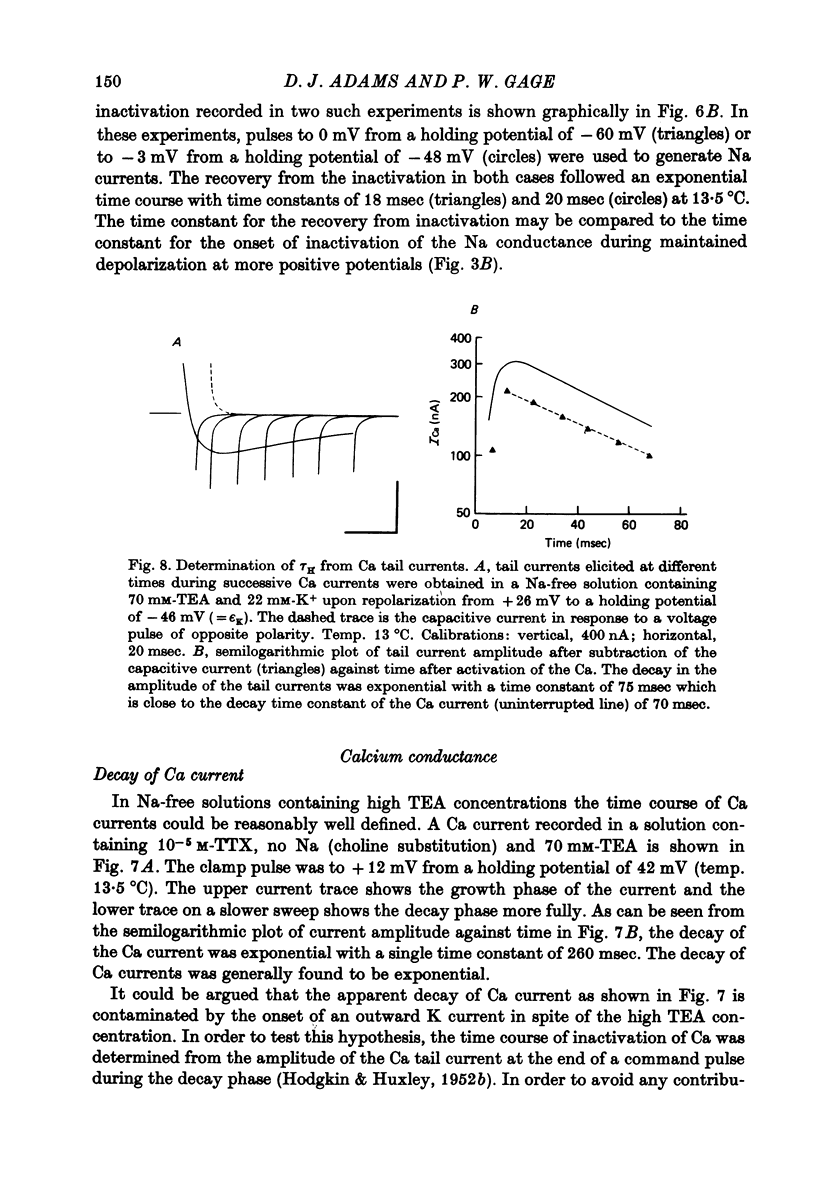
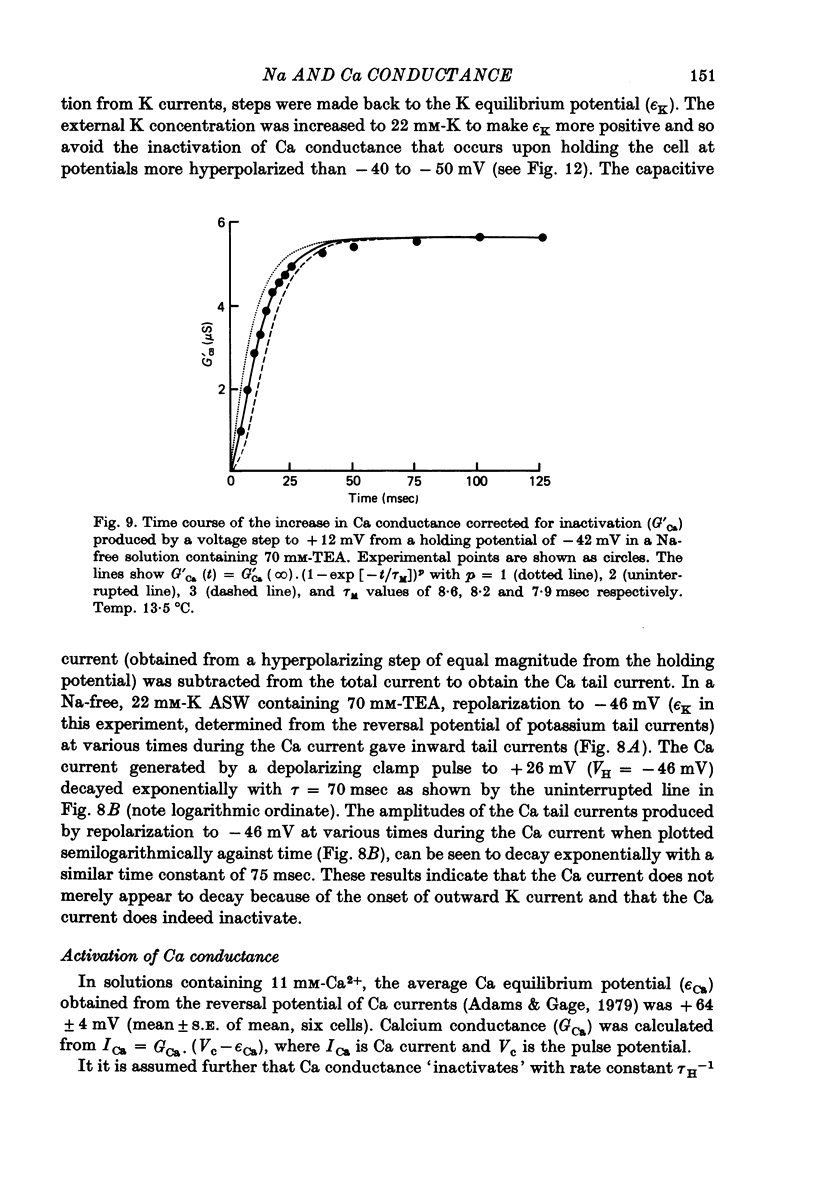
Abstract
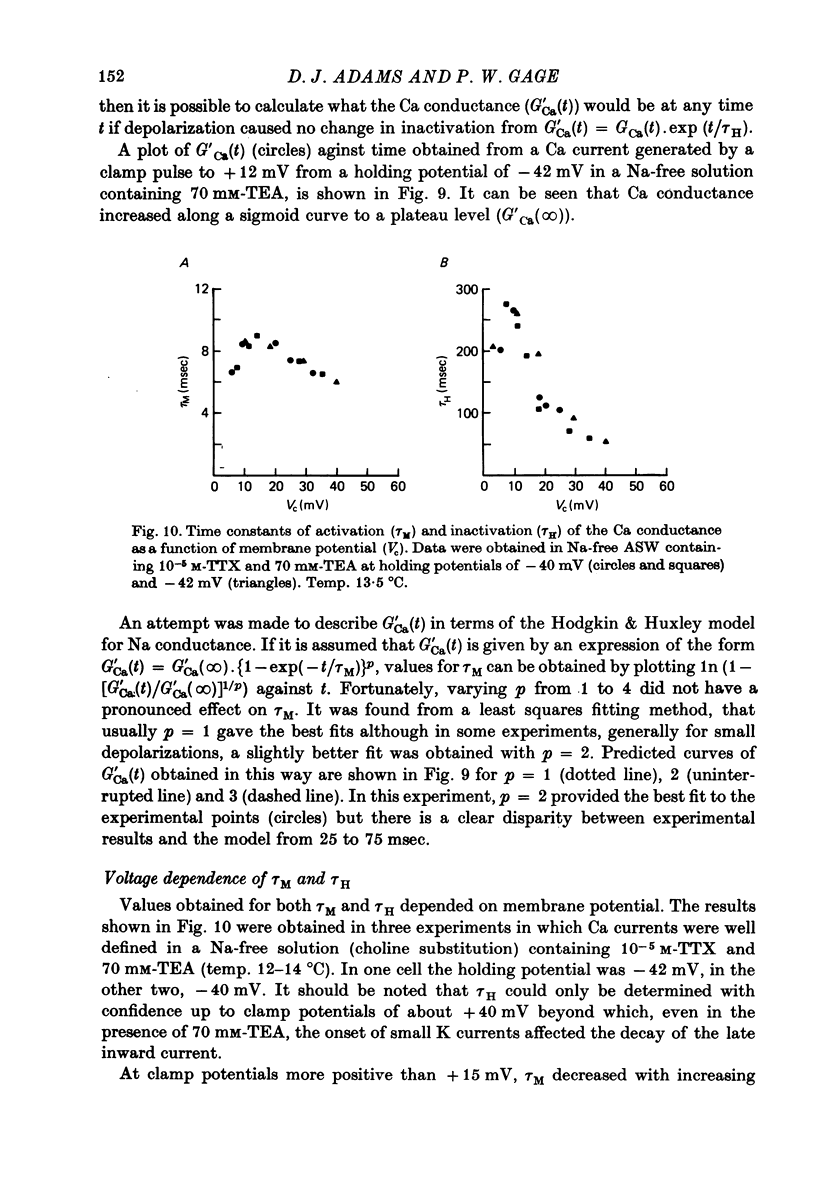
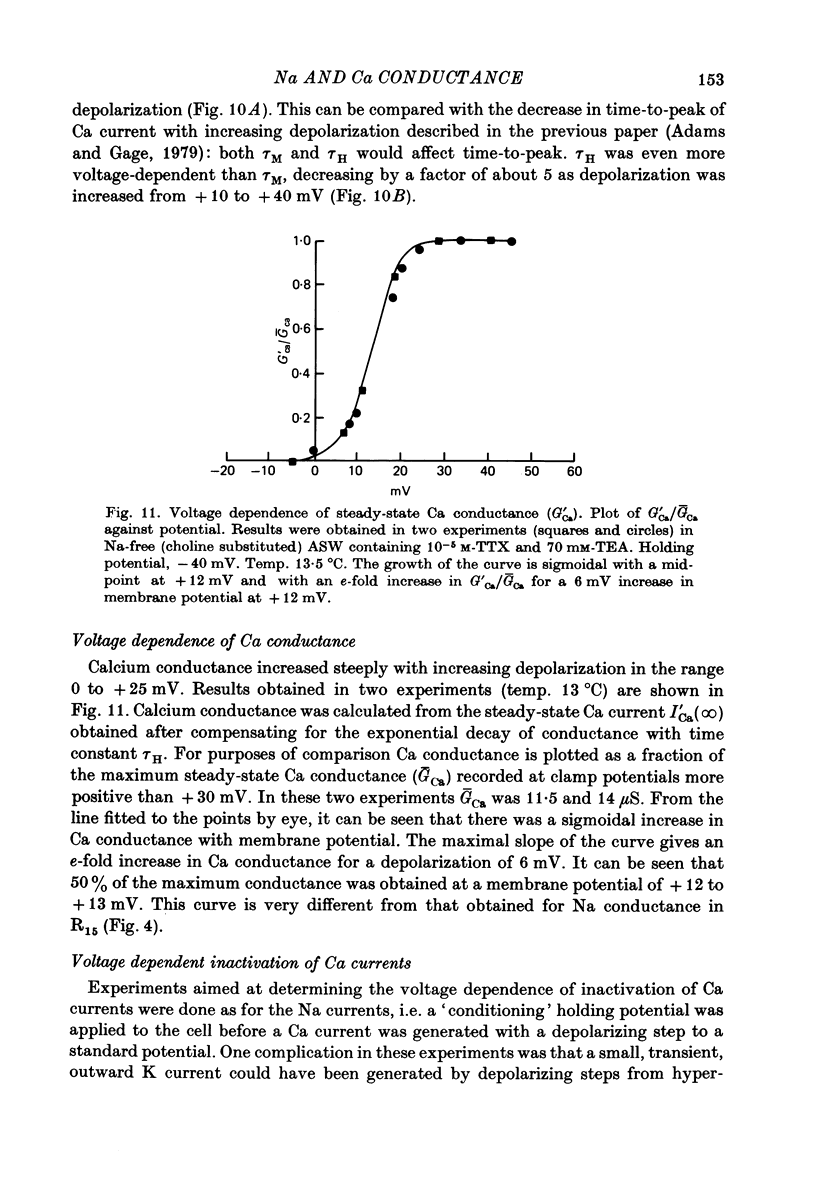
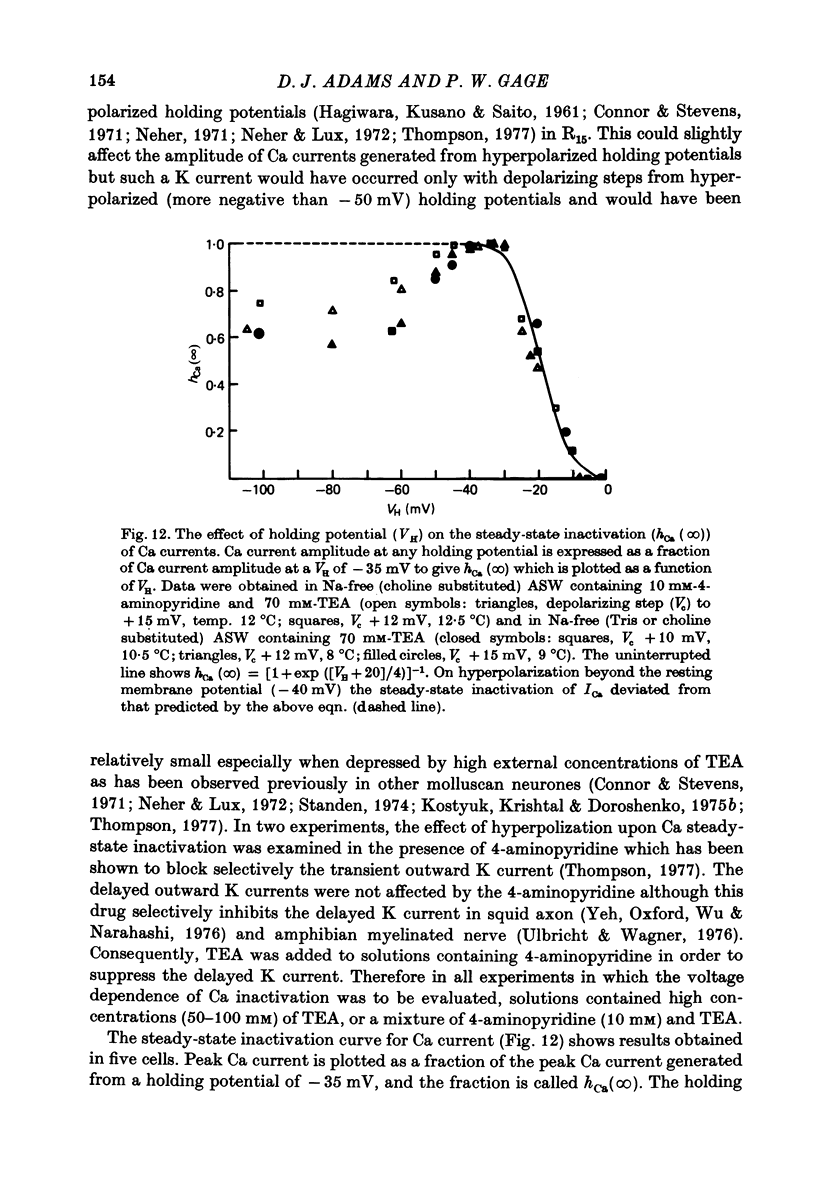
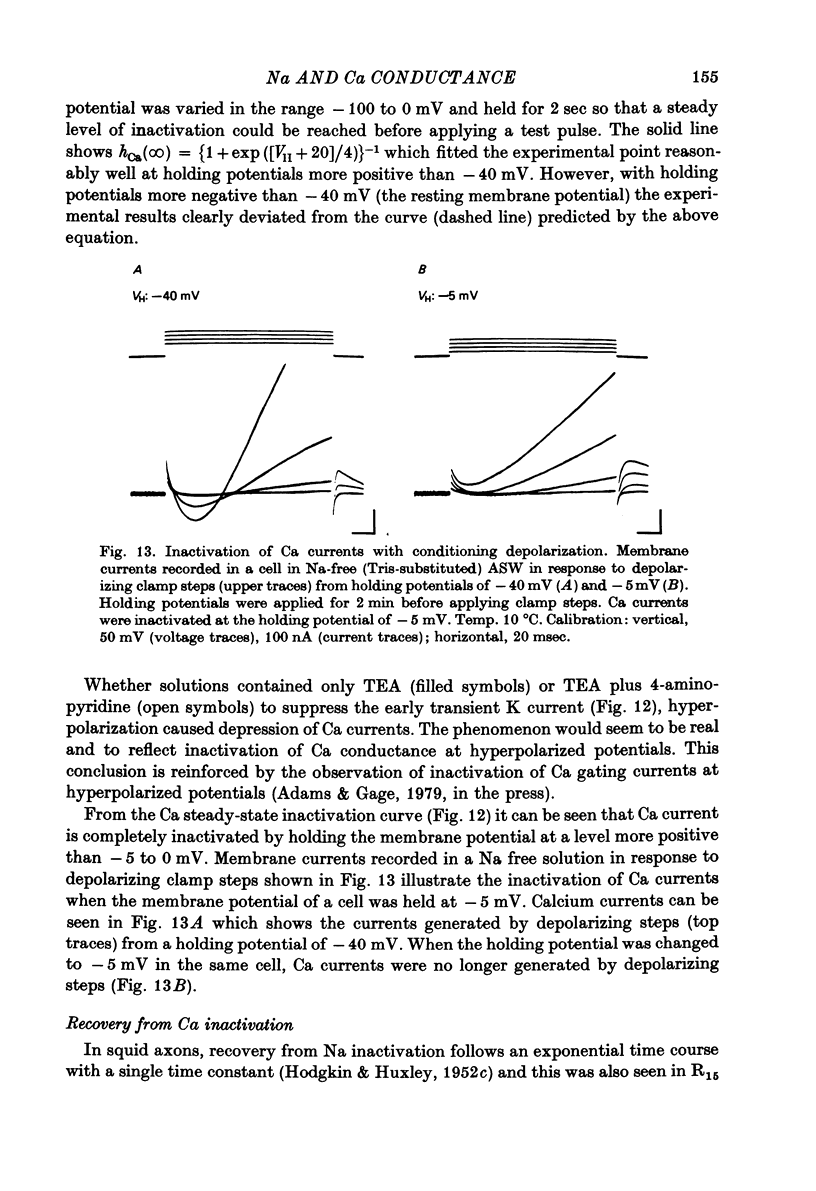
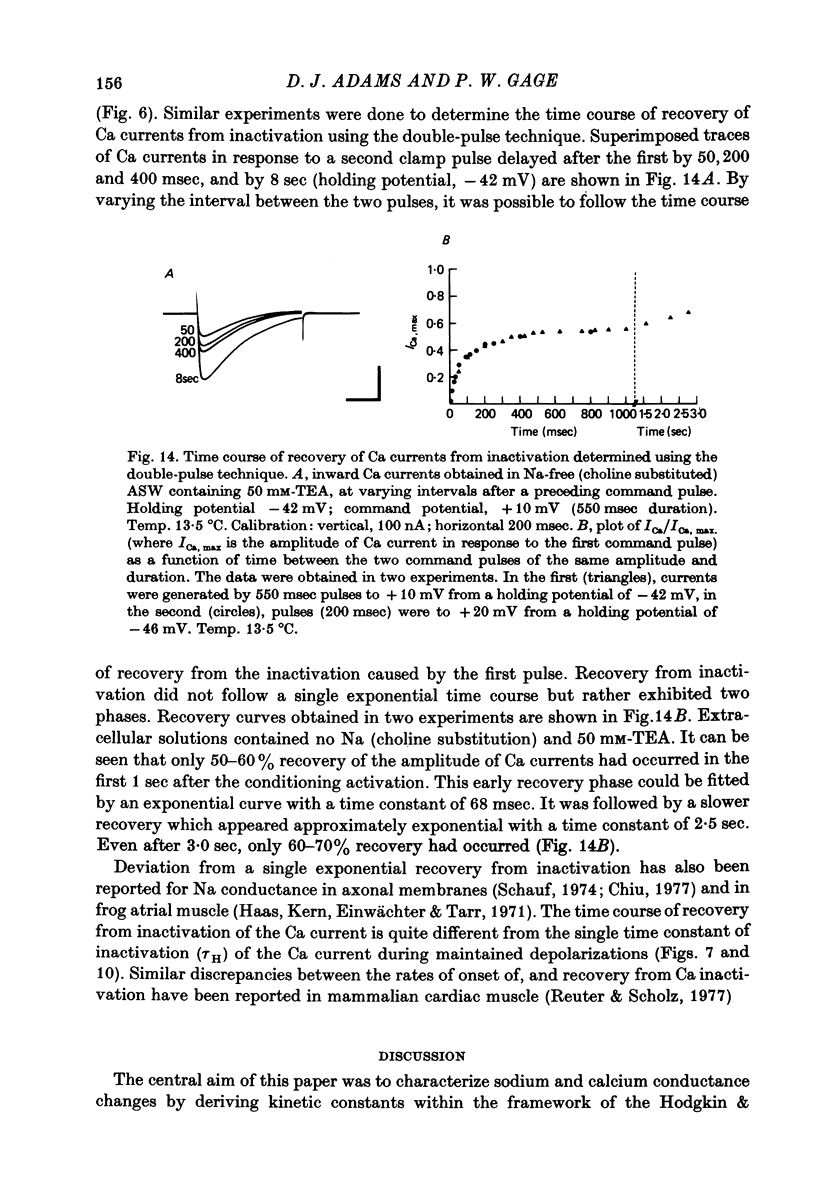
1. The time course and voltage dependence of Na and Ca conductance changes produced by depolarization of the soma of the neurone R15 in the abdominal ganglion of Aplysia juliana were examined at temperatures of 10--14 degrees C. 2. During a maintained depolarization, Na currents turned on then decayed (inactivated). Inactivation was exponential with time constant tauh. Activation (after correction for inactivation) was reasonably well described by the expression G'Na(t) = G'Na (infinity) (1 - exp [-t/taum])3 over a wide range of potentials. 3. taum and tauh were both voltage dependent. In the range -20 to +40 mV, taum varied from 5 to 0.5 msec and tauh from 25 to 8 msec (13.5 degrees C). Steady-state Na conductance (corrected for inactivation) was voltage dependent also, increasing sigmoidally with depolarization to a maximum of 25--30 muS at +10 to +20 mV. Half-maximal Na conductance occurred at a membrane potential of -8 mV and from -15 to -5 mV, a 5 mV change in membrane potential produced an e-fold change in steady-state Na conductance. 4. Steady-state inactivation of Na conductance (hNa(infinity)) was voltage dependent with half-inactivation occurring at a membrane potential of -32 mV. Recovery from Na inactivation followed an exponential time course with a voltage-dependent time constant. 5. During a maintained depolarization Ca currents activated then decayed (inactivated) more slowly than Na currents. The decay was exponential with time constant tauH. The decay of Ca current was not an artifact porduced by an outward current. The amplitude of calcium tail currents, produced by voltage steps back to epsilonK at different times during the decay of ICa, decayed also with a time constant close to tauH. 6. Ca conductance (after correction for inactivation) could be described approximately by the expression G'Ca(t) = G'Ca(infinity) (1 - exp [-t/tauM])p but it was necessary to vary p from 1 to 2 at different potentials. No value of p gave as good a fit to this model as that obtained for Na currents. 7. taum and tauH were voltage dependent. In the range of potentials from 0 to +60 mV, tauM varied from 9 to 5 msec and tauH from 300 to 50 msec (13.5 degrees C). Steady-state Ca conductance (corrected for inactivation) was voltage dependent also, increasing sigmoidally with depolarization to a maximum of 10--15 muS at +30 to +40 mV. Half-maximal Ca conductance occurred at a membrane potential of +12 mV, and from +10 to +20 mV a 6 mV change in membrane potential produced an e-fold change in Ca conductance. 8. Steady-state inactivation of Ca conductance (hCa(infinity)) varied with holding potential (VH). Half-inactivation occurred with depolarization to -20 mV. At potentials more negative than -40 mV, hCa(infinity) was less than at -40 mV, i.e. hyperpolarization produced Ca 'inactivation'. 9...
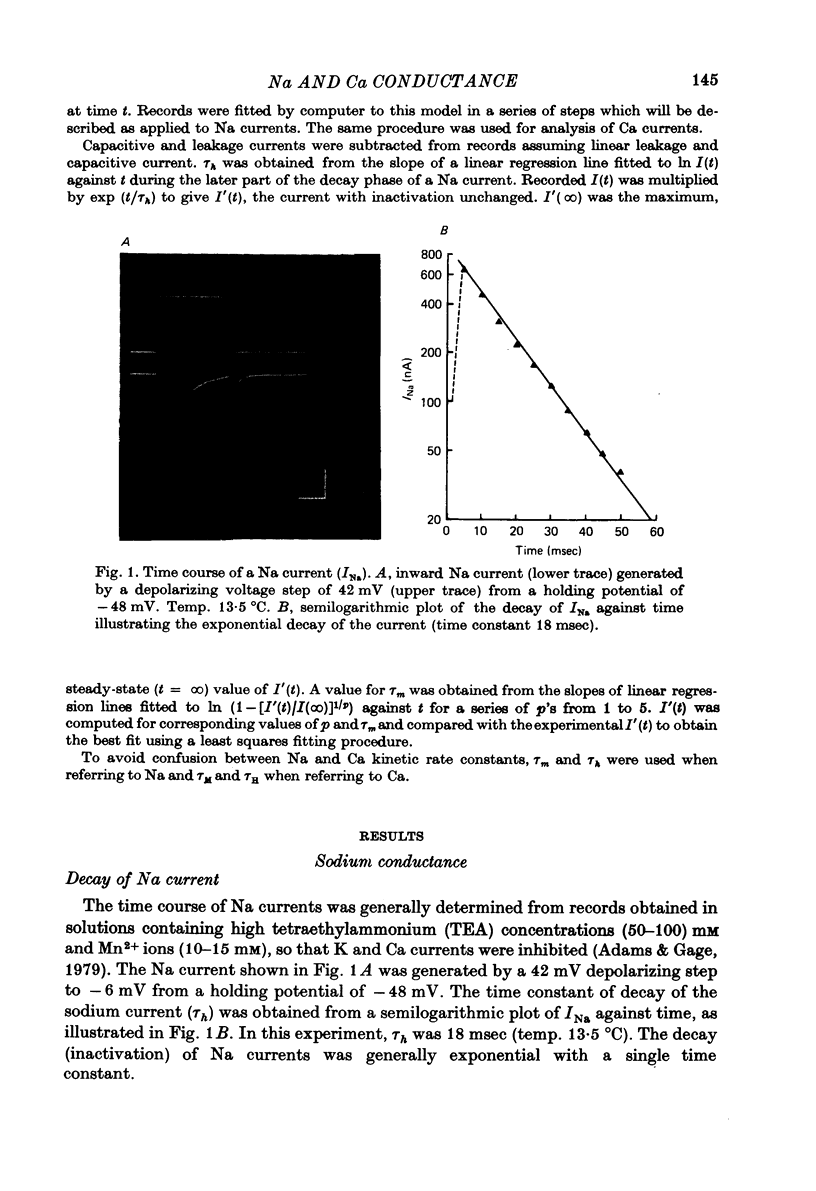
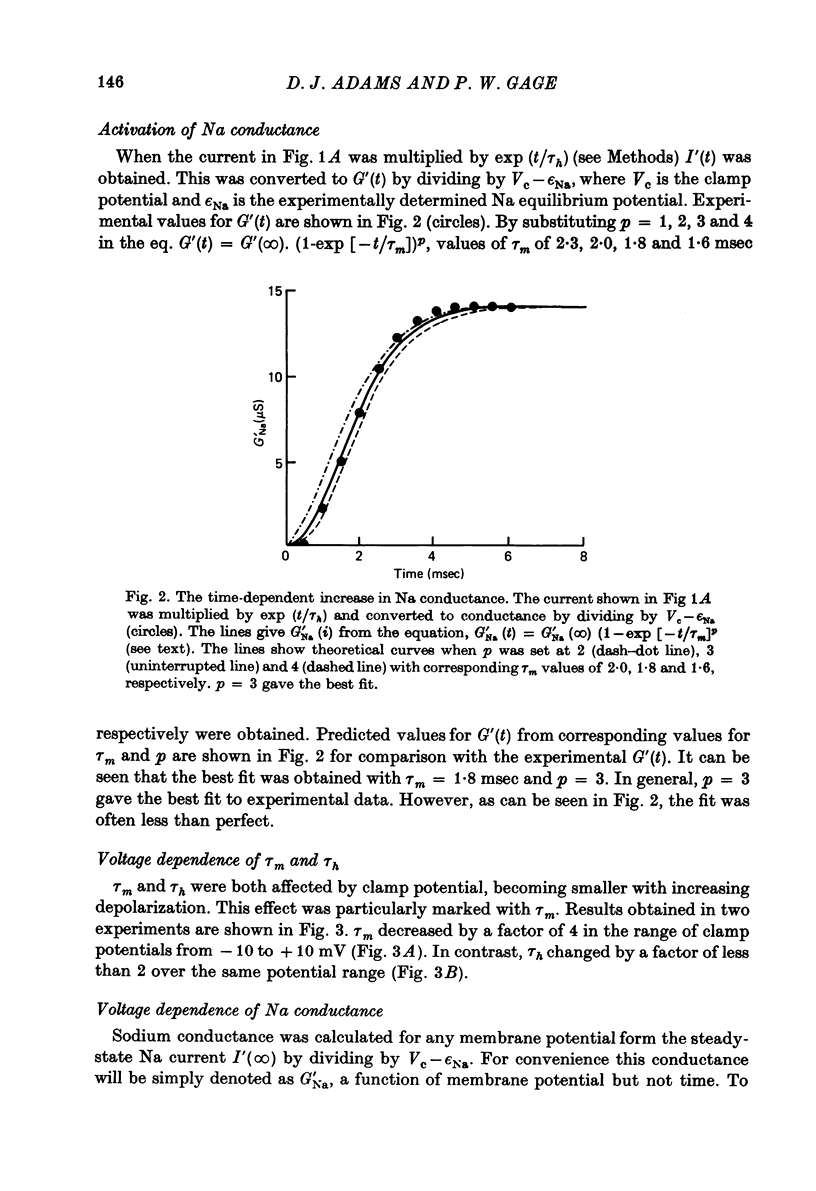
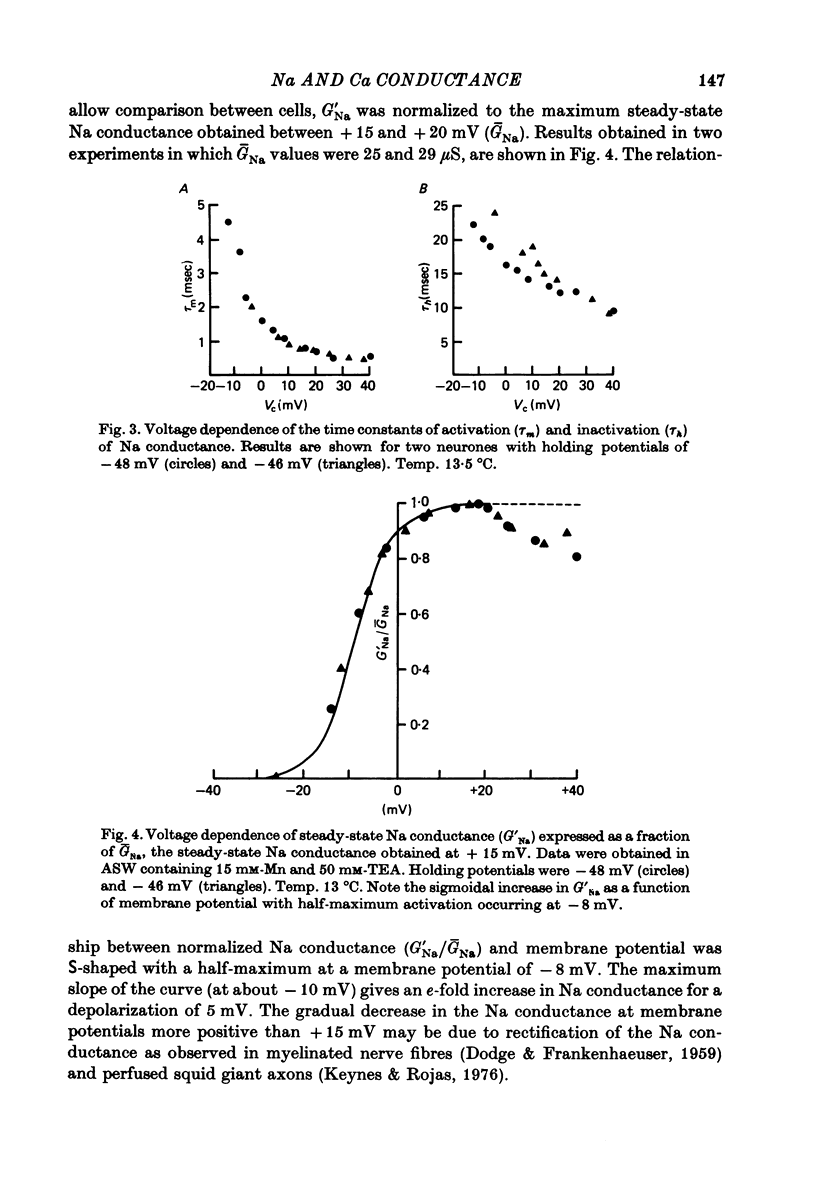
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W. Gating currents associated with sodium and calcium currents in an Aplysia neuron. Science. 1976 May 21;192(4241):783–784. doi: 10.1126/science.1265479. [DOI] [PubMed] [Google Scholar]
- Adams D. J., Gage P. W. Ionic currents in response to membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:115–141. doi: 10.1113/jphysiol.1979.sp012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Voltage-dependent changes in the permeability of nerve membranes to calcium and other divalent cations. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):389–409. doi: 10.1098/rstb.1975.0018. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Bennett M. V. Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. J Gen Physiol. 1977 Feb;69(2):145–182. doi: 10.1085/jgp.69.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D., Ridgway E. B. Voltage-dependent facilitation of Ca2+ entry in voltage-clamped, aequorin-injected molluscan neurons. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1748–1752. doi: 10.1073/pnas.74.4.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hencek M., Zachar J. Calcium currents and conductances in the msucle membrane of the crayfish. J Physiol. 1977 Jun;268(1):51–71. doi: 10.1113/jphysiol.1977.sp011846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Gating in sodium channels of nerve. Annu Rev Physiol. 1976;38:139–152. doi: 10.1146/annurev.ph.38.030176.001035. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Komai Y., Matsukawa S., Satake M. Lipid composition of the nervous tissue of the invertebrates Aplysia kurodai (gastropod) and Cambarus clarki (arthropod). Biochim Biophys Acta. 1973 Sep 25;316(3):271–281. [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Ringham G. L. Synaptic transfer at a vertebrate central nervous system synapse. J Physiol. 1975 Oct;251(2):409–426. doi: 10.1113/jphysiol.1975.sp011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Differential action of TEA + on two K + -current componentss of a molluscan neurone. Pflugers Arch. 1972;336(2):87–100. doi: 10.1007/BF00592924. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimahara T., Tauc L. Multiple interneuronal afferents to the giant cells in Aplysia. J Physiol. 1975 May;247(2):299–319. doi: 10.1113/jphysiol.1975.sp010933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Voltage-clamp studies of the calcium inward current in an identified snail neurone: comparison with the sodium inward current. J Physiol. 1975 Jul;249(2):253–268. doi: 10.1113/jphysiol.1975.sp011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]