Abstract
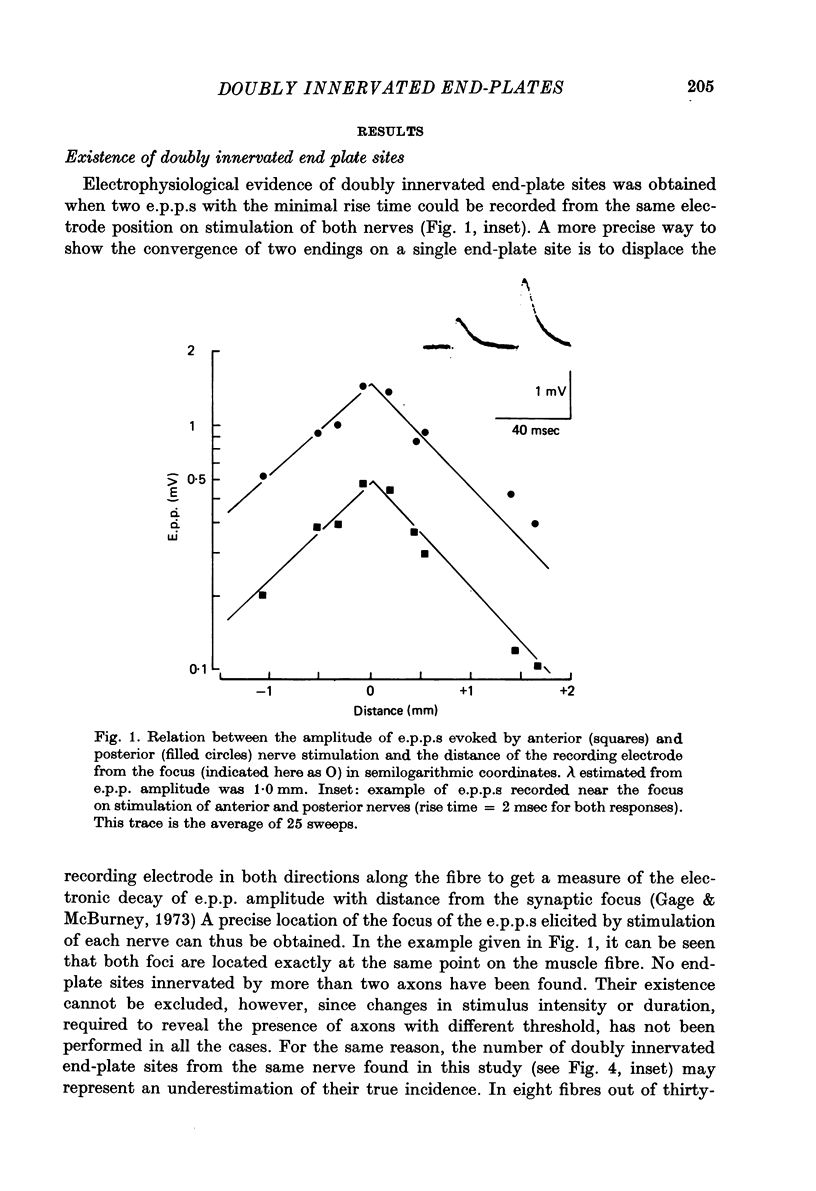
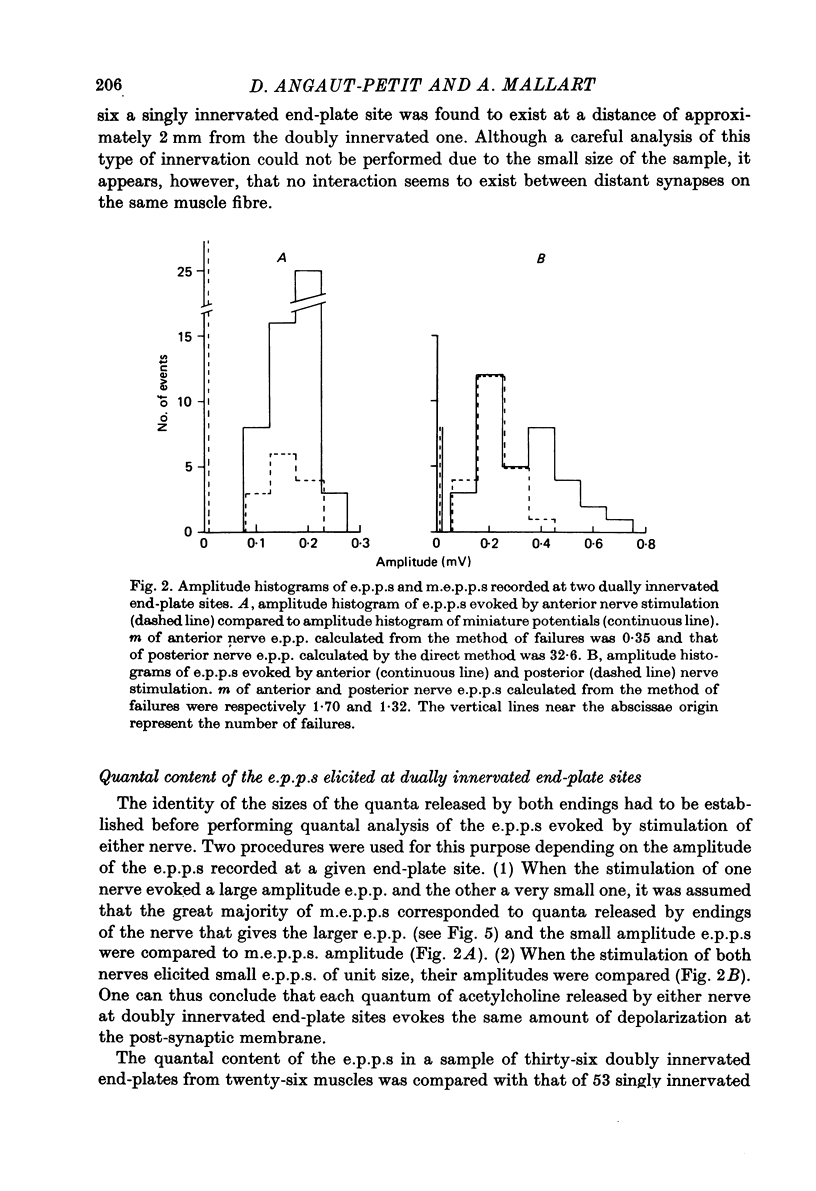
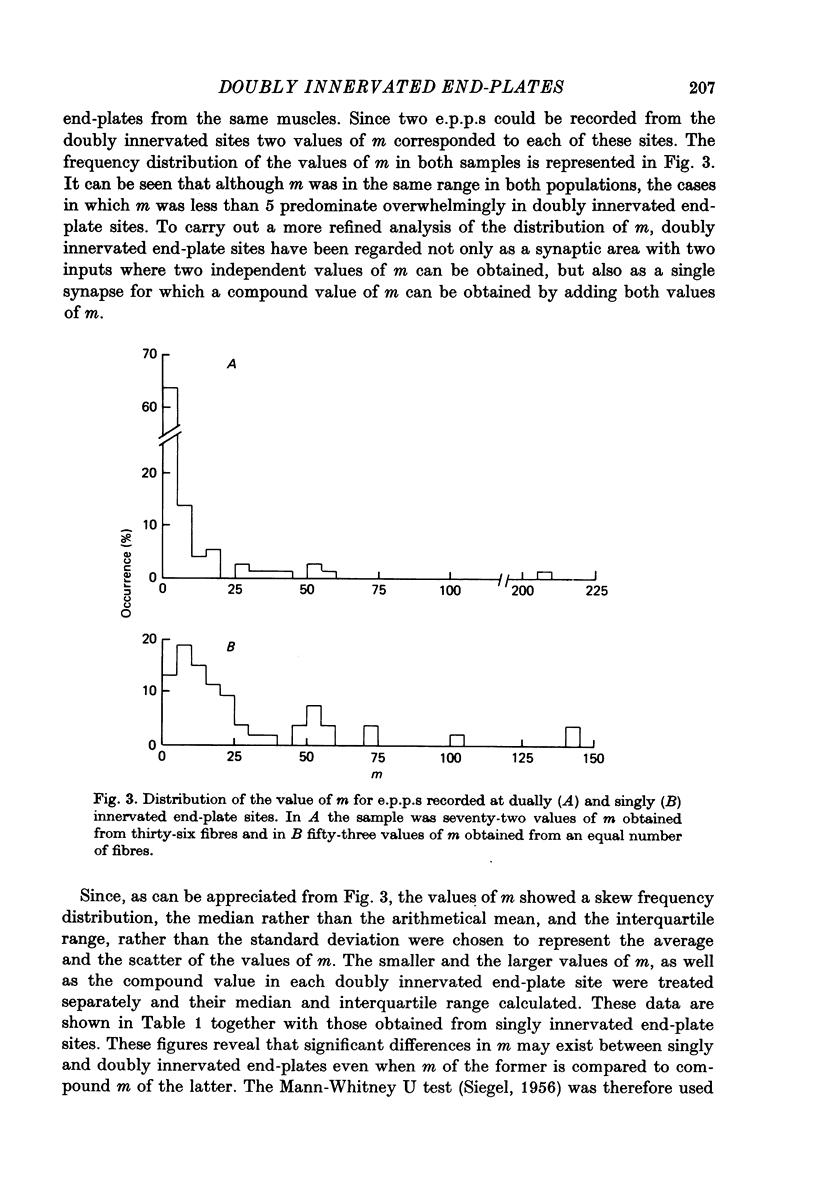
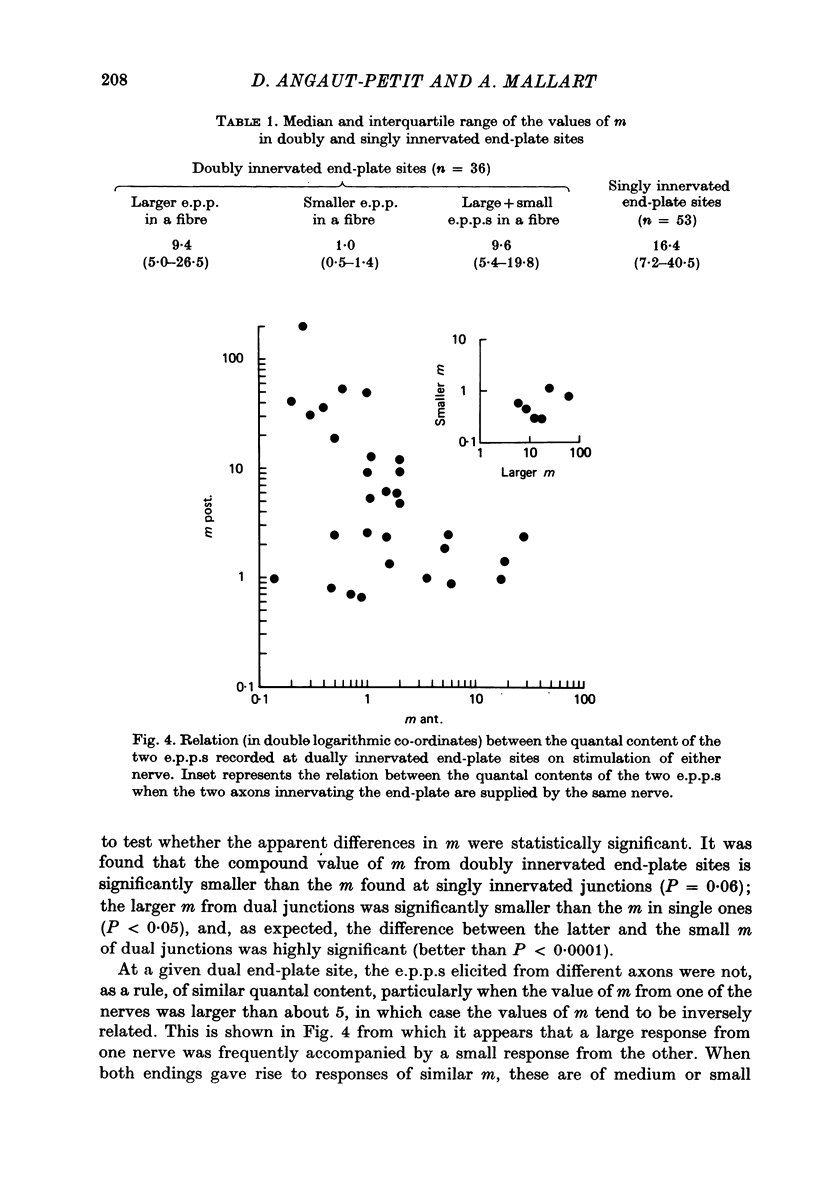
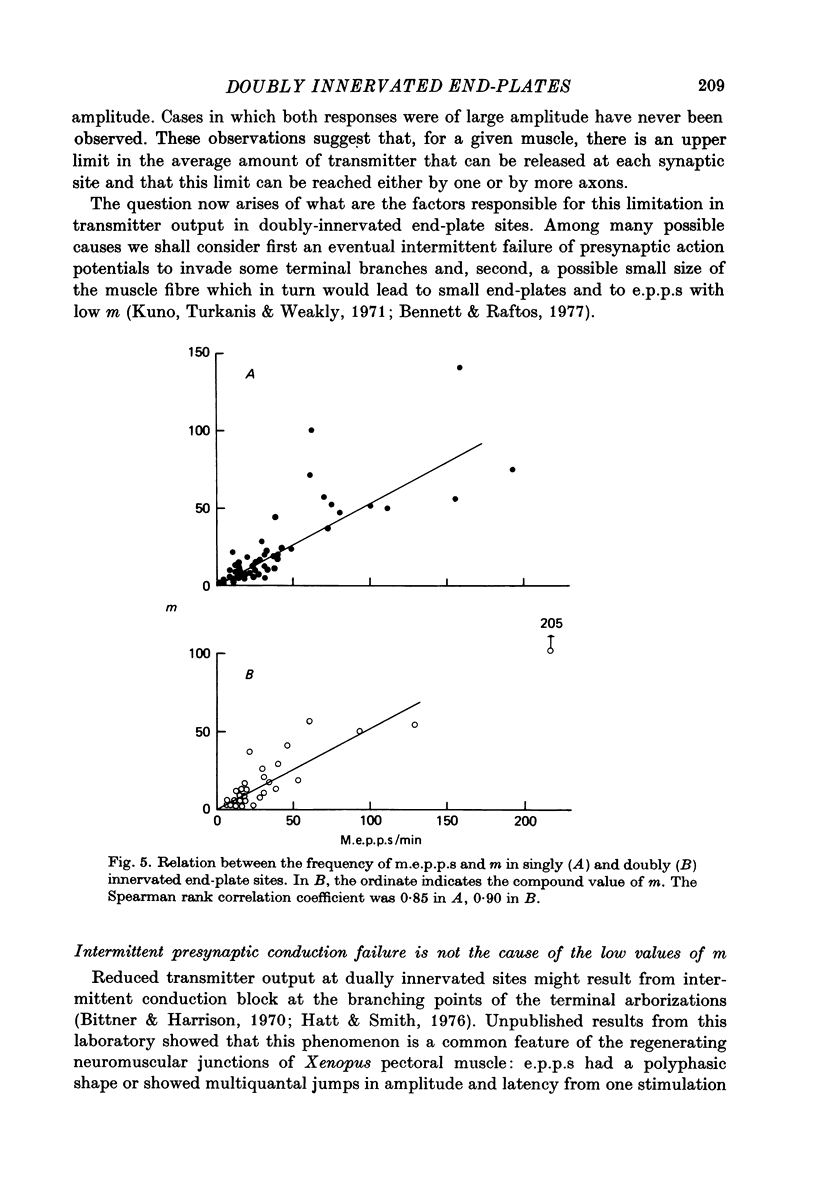
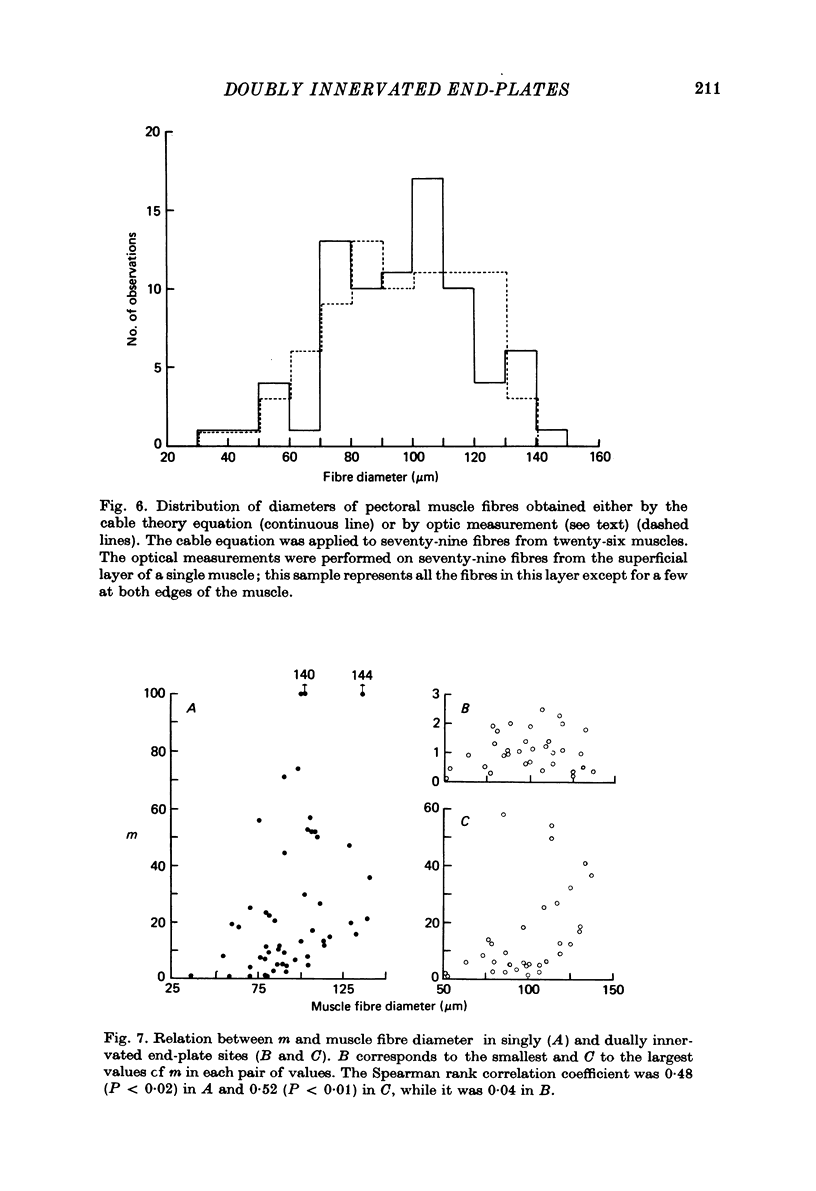
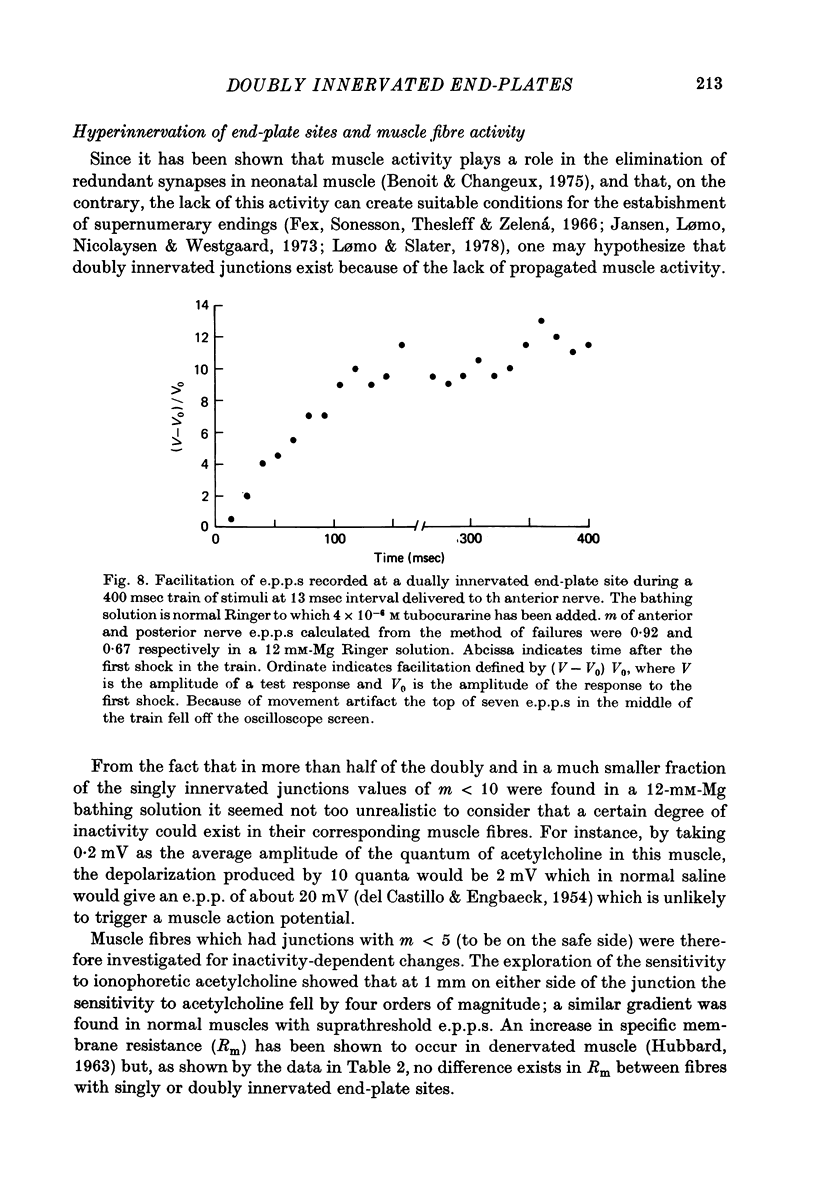
1. Electrophysiological study of dually innervated end-plate sites was carried out in Xenopus laevis pectoral muscle fibres. End-plate potentials (e.p.p.s) and miniature end-plate potentials (m.e.p.p.s) have been recorded in Mg-blocked preparations. 2. The mean quantal content (m) of each e.p.p. at dually innervated end-plates was significantly smaller than the corresponding values obtained at singly innervated ones. At a given doubly innervated end-plate site the values of m tended to be inversely related, so that the compound value of m (obtained by adding them) was in the same range as that found in singly innervated junctions. These findings were taken to suggest the existence of an upper limit in the average amount of transmitter released at a synaptic site. 3. It was found that neither intermittent presynaptic conduction block, nor particular muscle fibre properties could account for the low values of m in dual end plates. The small size of the nerve terminals appears to be the most likely explanation. 4. The sensitivity to acetylcholine and muscle fibre electrical properties were investigated; no differences were found between fibres with sub- or suprathreshold e.p.p.s. 5. The nature of the factors responsible for this presumed small size of the nerve endings (competition between nerve endings for a limited synaptic space on the muscle membrane or reciprocal interaction between closely located terminals) as well as the possible origins of polyinnervation are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B. Upper and lower bounds on the non-linearity of summation of end-plate potentials. J Theor Biol. 1976 Nov;63(1):217–224. doi: 10.1016/0022-5193(76)90093-x. [DOI] [PubMed] [Google Scholar]
- Aguilar C. E., Bisby M. A., Cooper E., Diamond J. Evidence that axoplasmic transport of trophic factors is involved in the regulation of peripheral nerve fields in salamanders. J Physiol. 1973 Oct;234(2):449–464. doi: 10.1113/jphysiol.1973.sp010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Bittner G. D., Harrison J. A reconsideration of the Poisson hypothesis for transmitter release at the crayfish neuromuscular junction. J Physiol. 1970 Jan;206(1):1–23. doi: 10.1113/jphysiol.1970.sp008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Diamond J., Turner C. The effects of nerve section and of colchicine treatment on the density of mechanosensory nerve endings in salamander skin. J Physiol. 1977 Jan;264(3):725–749. doi: 10.1113/jphysiol.1977.sp011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex S., Sonesson B., Thesleff S., Zelená J. Nerve implants in botulinum poisoned mammalian muscle. J Physiol. 1966 Jun;184(4):872–882. doi: 10.1113/jphysiol.1966.sp007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B., Letinsky M. S. Mutual repression of synaptic efficacy by pairs of foreign nerves innervating frog skeletal muscle. Nature. 1977 Jan 27;265(5592):368–370. doi: 10.1038/265368a0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Fate of interrupted nerve-fibres regenerating into partially denervated muscles. Aust J Exp Biol Med Sci. 1951 May;29(3):211–219. doi: 10.1038/icb.1951.25. [DOI] [PubMed] [Google Scholar]
- HUBBARD S. J. The electrical constants and the component conductances of frog skeletal muscle after denervation. J Physiol. 1963 Mar;165:443–456. doi: 10.1113/jphysiol.1963.sp007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Lomo T., Nicolaysen K., Westgaard R. H. Hyperinnervation of skeletal muscle fibers: dependence on muscle activity. Science. 1973 Aug 10;181(4099):559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Kuffler D., Thompson W., Jansen J. K. The elimination of synapses in multiply-innervated skeletal muscle fibres of the rat: dependence on distance between end-plates. Brain Res. 1977 Dec 16;138(2):353–358. doi: 10.1016/0006-8993(77)90752-1. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S. Acetylcholine sensitivity changes in tadpole tail muscle fibers innervated by developing motor neurons. J Neurobiol. 1975 Nov;6(6):609–617. doi: 10.1002/neu.480060607. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of acetylcholine sensitivity and synapse formation by muscle activity. J Physiol. 1978 Feb;275:391–402. doi: 10.1113/jphysiol.1978.sp012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magherini P. C., Precht W. Electrical properties of frog motoneurons in the in situ spinal cord. J Neurophysiol. 1976 May;39(3):459–473. doi: 10.1152/jn.1976.39.3.459. [DOI] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Bastian J. Double sucrose-gap method applied to single muscle fiber of Xenopus laevis. J Gen Physiol. 1974 Feb;63(2):235–256. doi: 10.1085/jgp.63.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., McMahan U. J. Altered patterns of innervation in frog muscle after denervation. J Neurocytol. 1976 Dec;5(6):719–730. doi: 10.1007/BF01181583. [DOI] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- VAN HARREVELD A., TACHIBANA S. Innervation and reinnervation of cricothyroid muscle in the rabbit. Am J Physiol. 1961 Dec;201:1199–1202. doi: 10.1152/ajplegacy.1961.201.6.1199. [DOI] [PubMed] [Google Scholar]
- Vigny M., Bon S., Massoulié J., Leterrier F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, torpedo, rat and chicken. Eur J Biochem. 1978 Apr 17;85(2):317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Magazanik L. G. Dual end-plate potentials at the single neuromuscular junction of the adult frog. Pflugers Arch. 1977 Apr 25;368(3):271–273. doi: 10.1007/BF00585207. [DOI] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]


