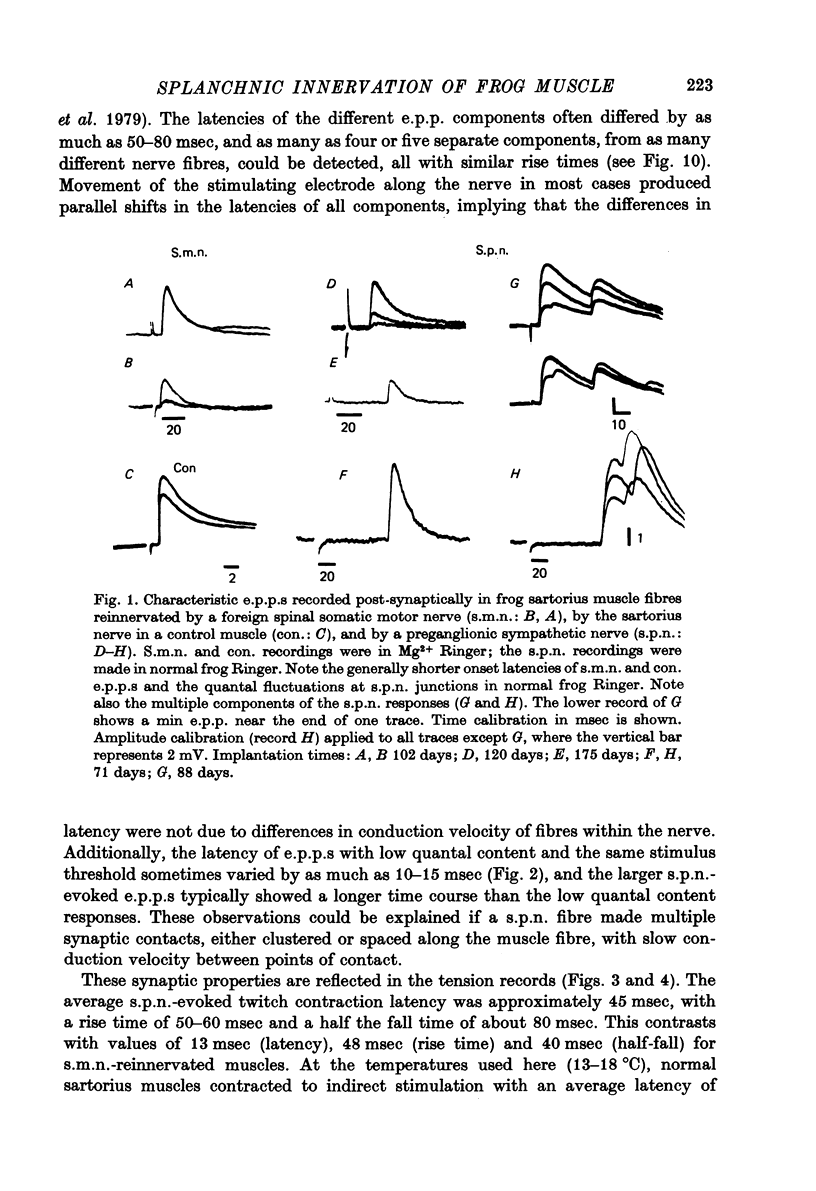
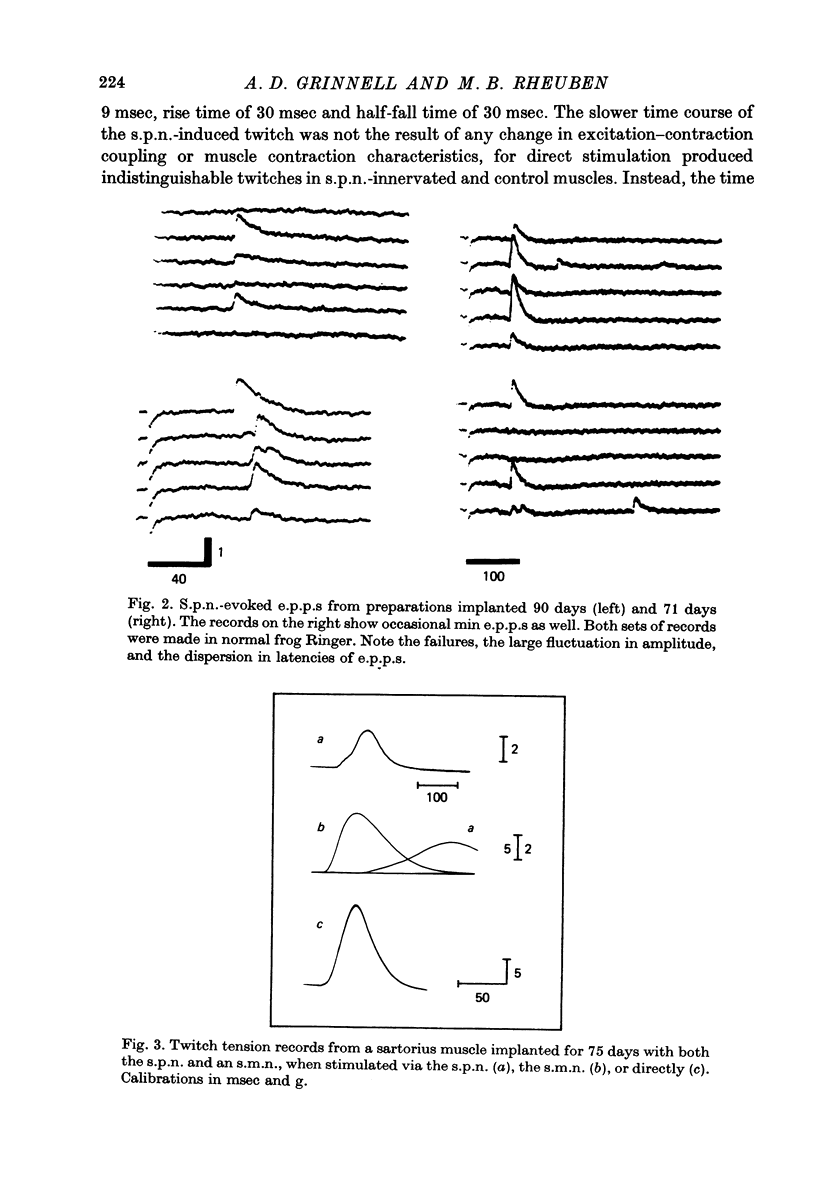
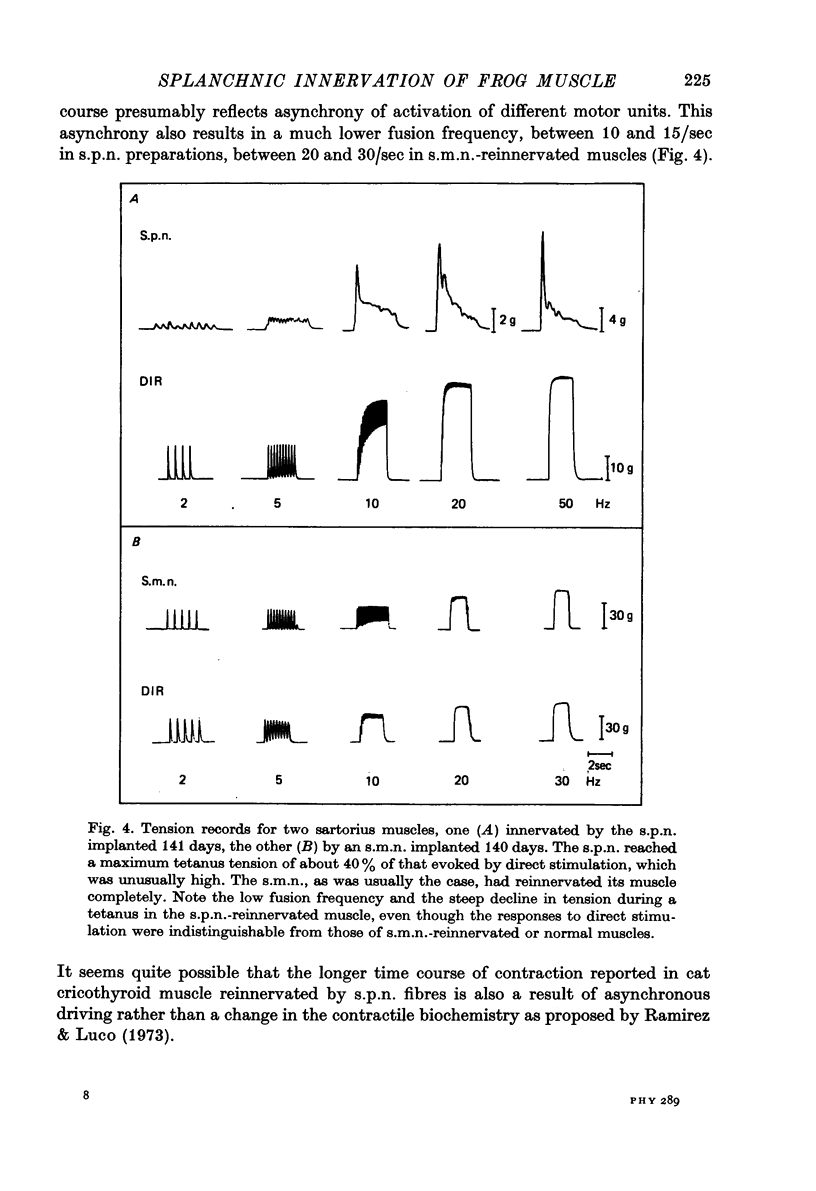
Abstract
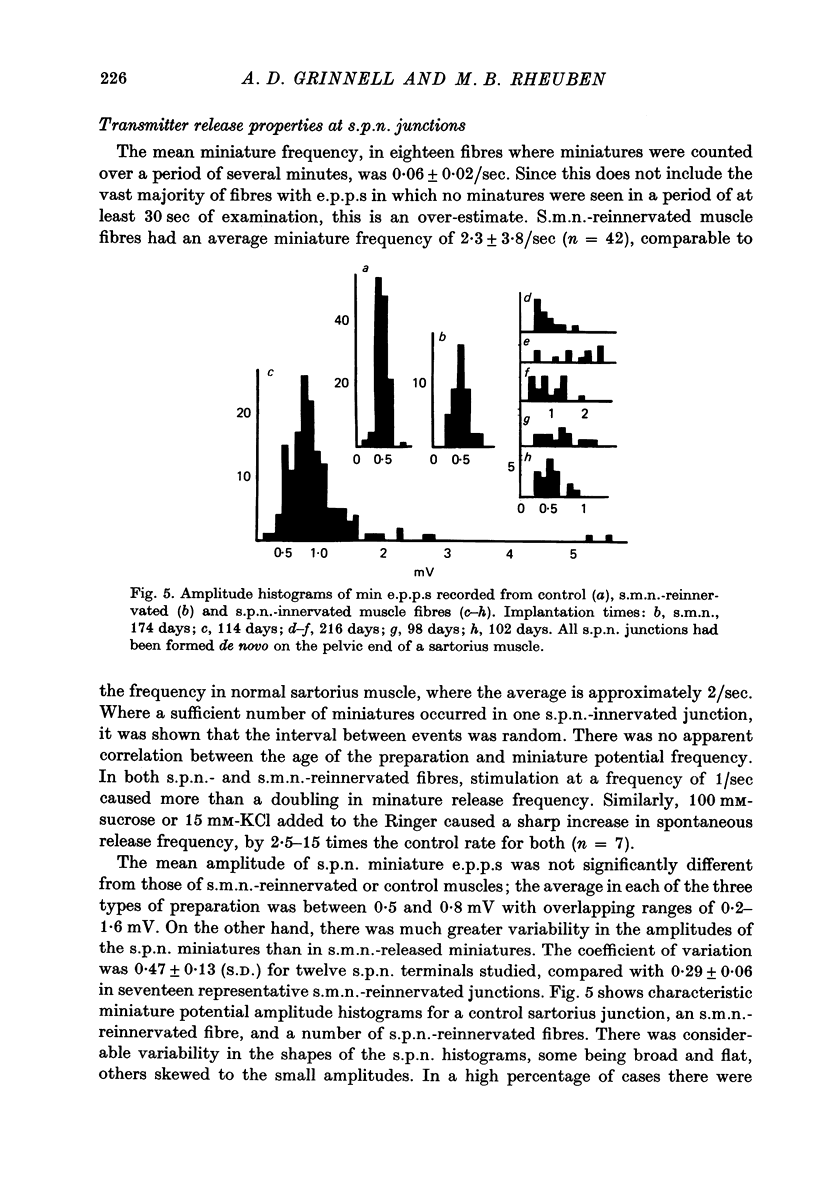
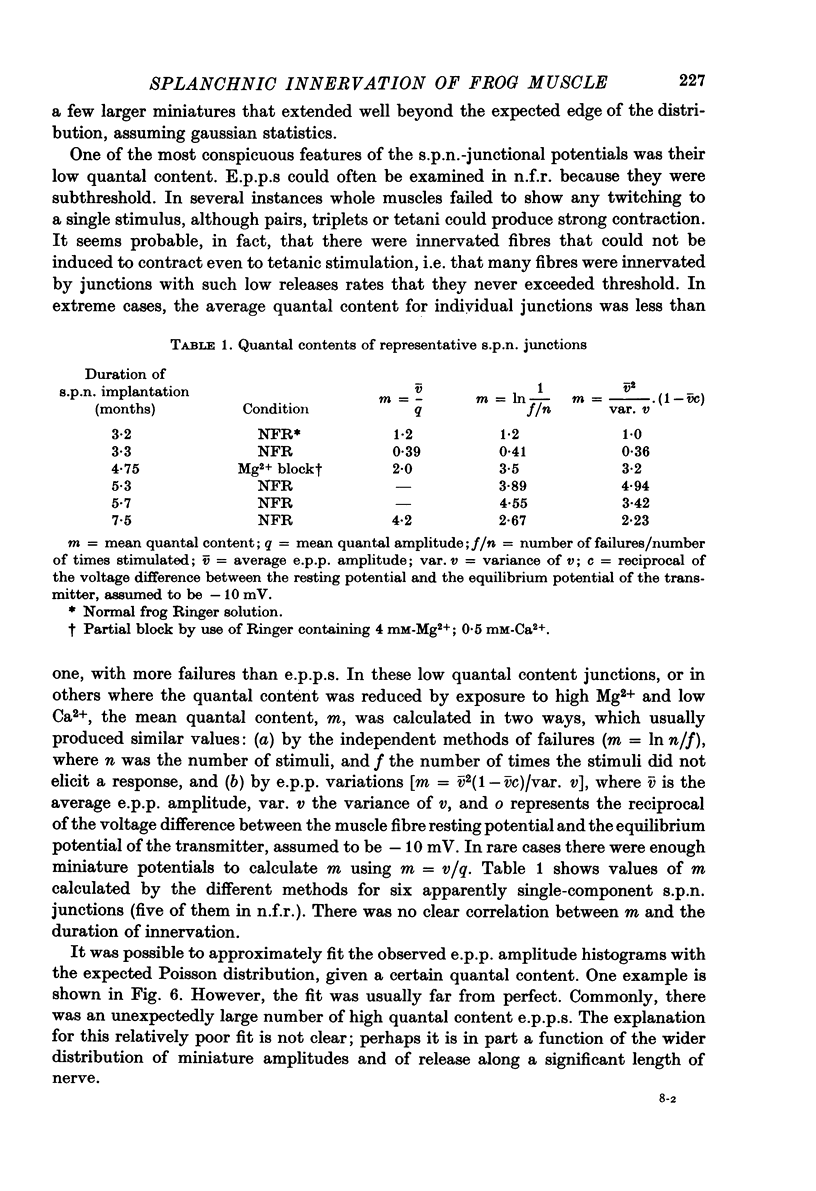
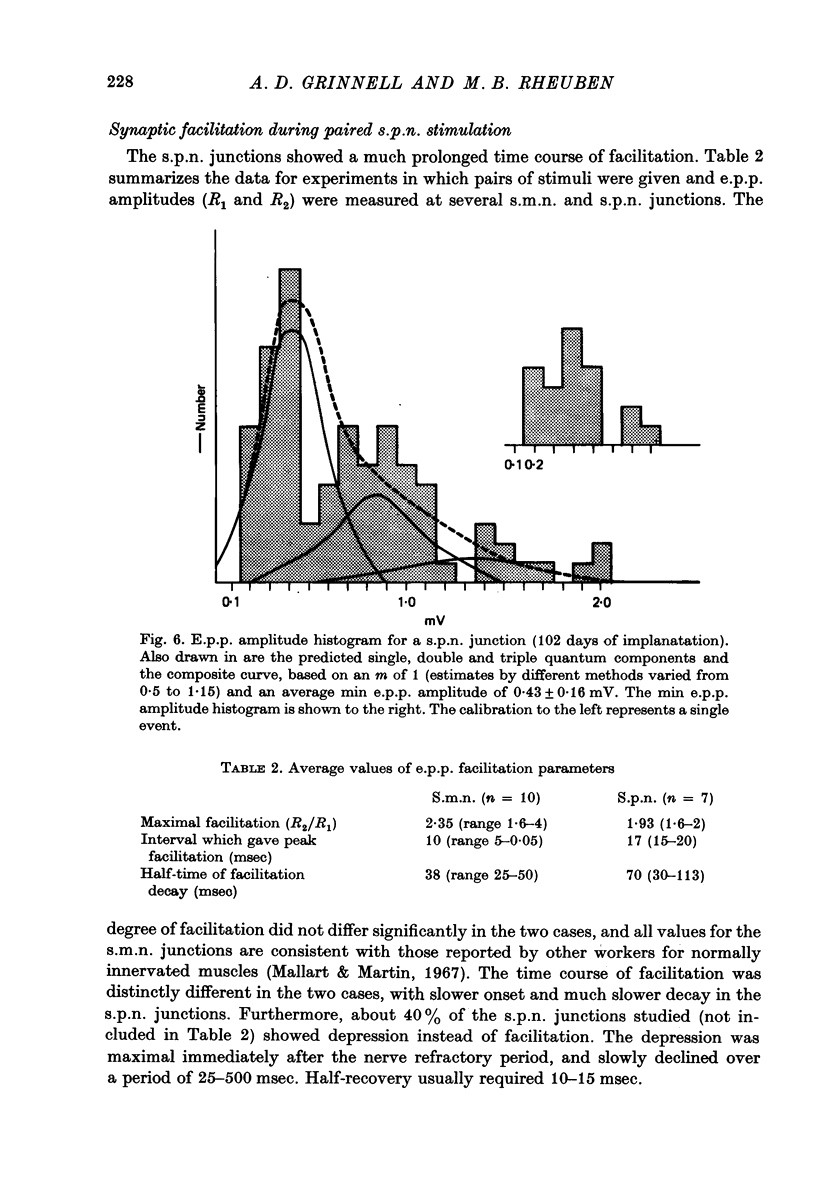
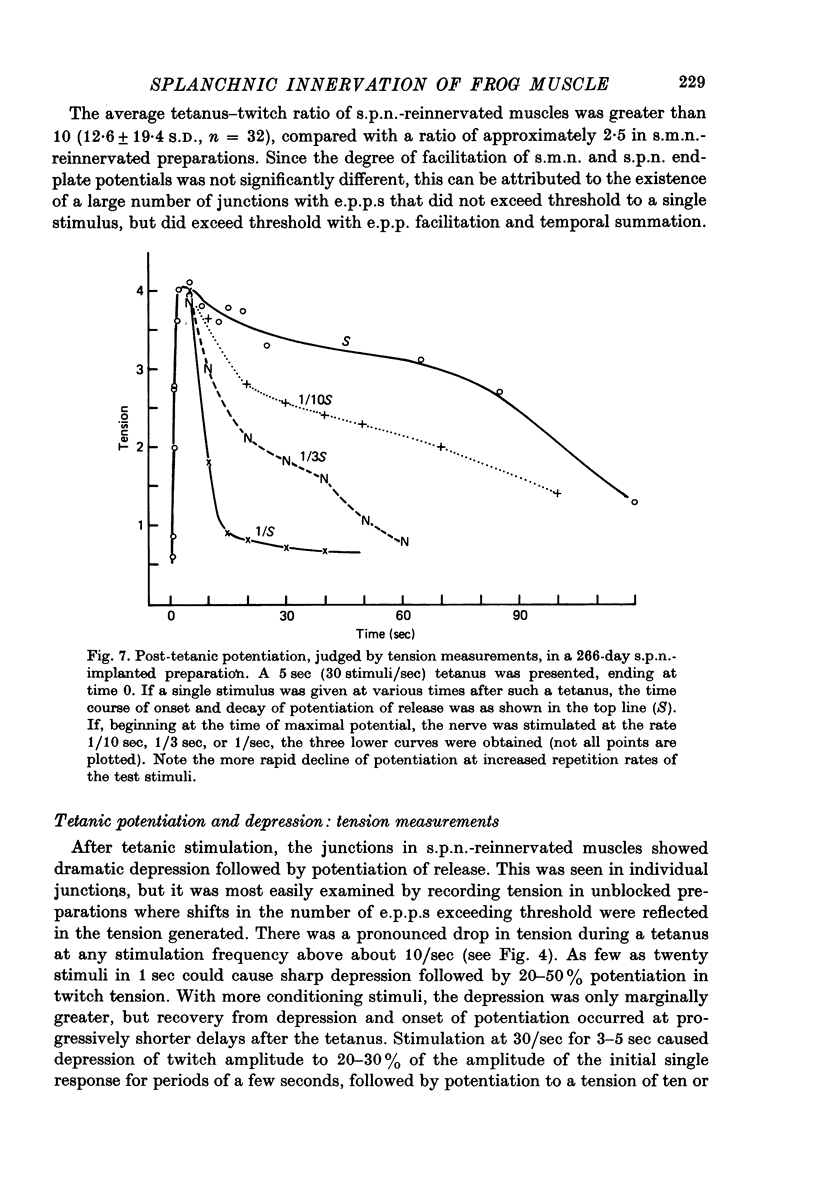
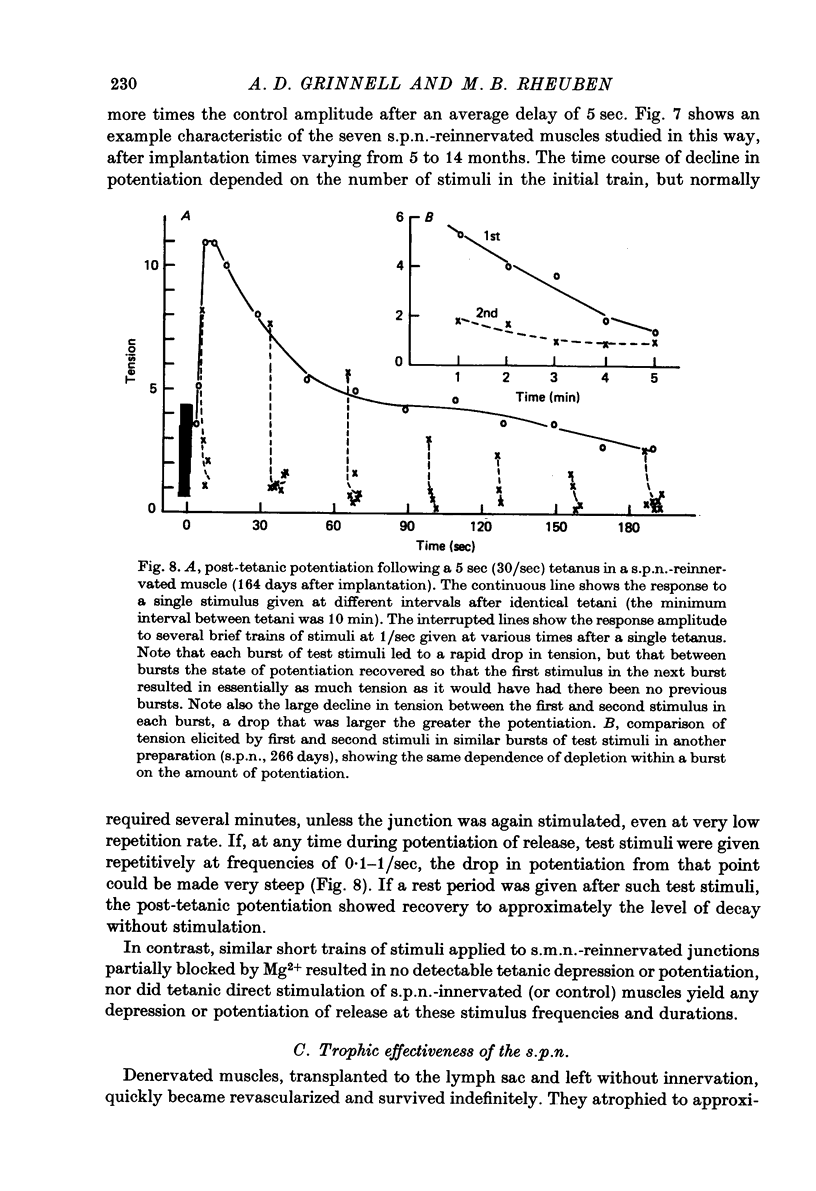
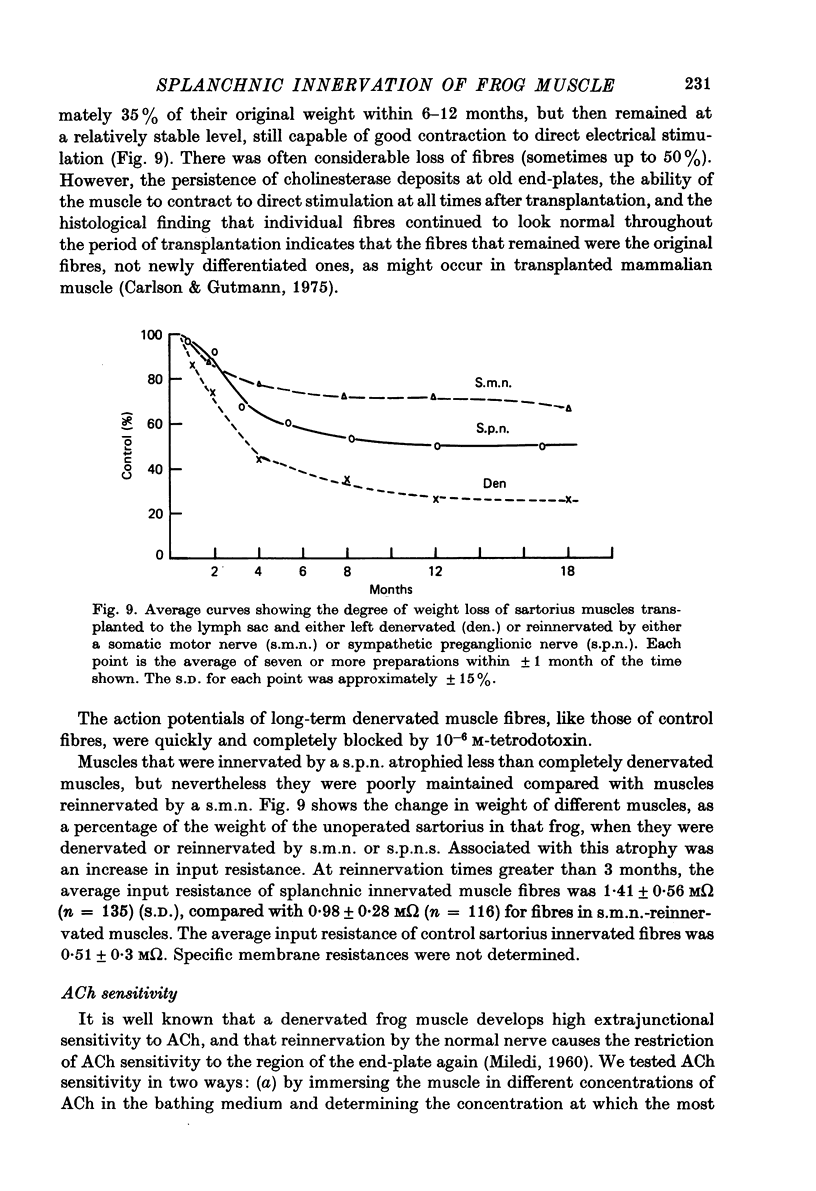
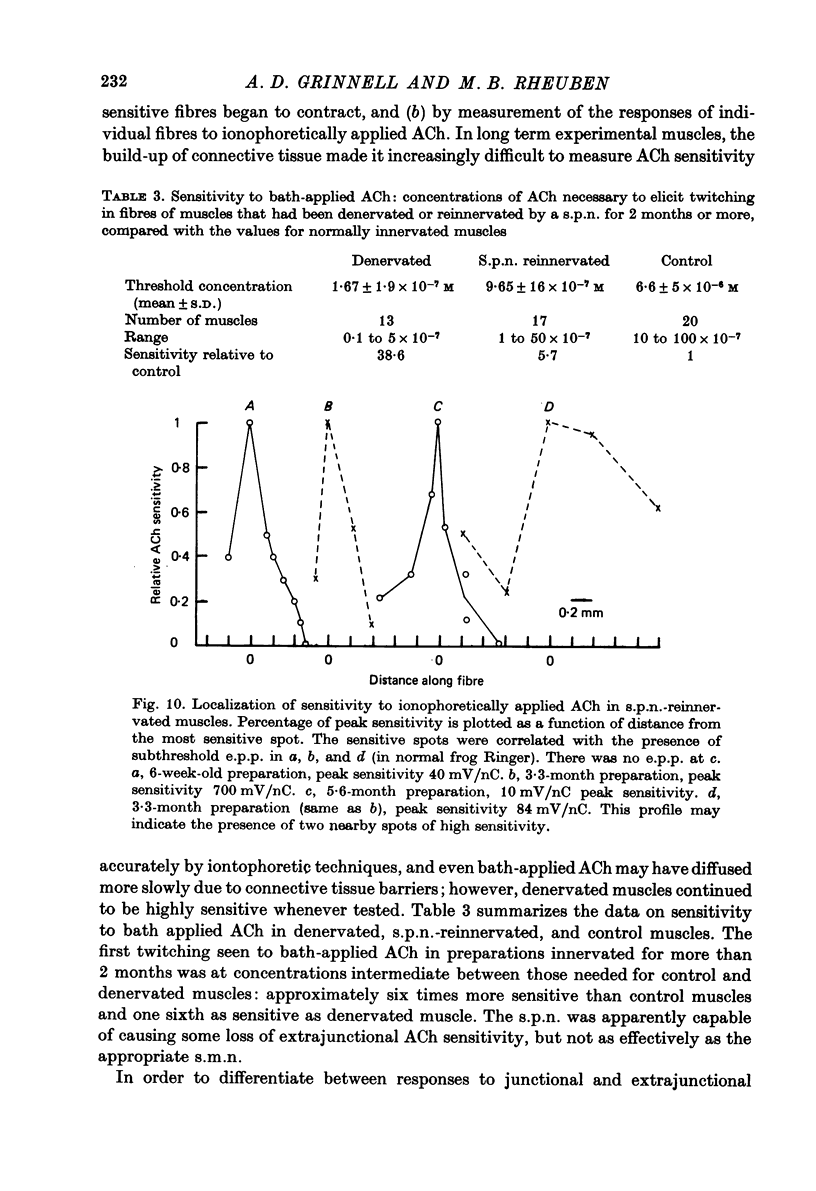
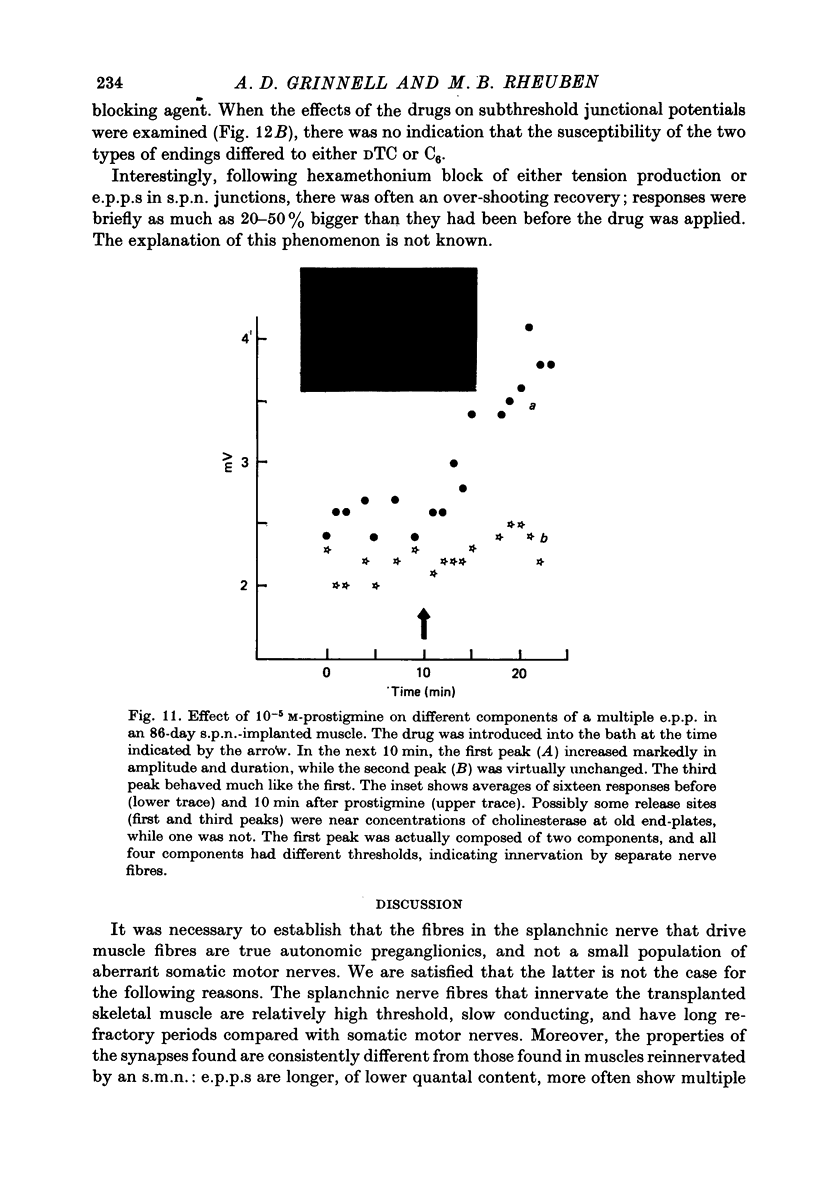
1. Frog sartorius muscles, newly denervated and transplanted to the lymph sac of the back, are reinnervated by implanted cholinergic nerves (spinal somatic motor nerves or the preganglionic sympathetic splanchnic nerve), but not by nerves). 2. Foreign somatic motor nerves (s.m.n.s) form synapses that resemble normal sartorius neuromuscular junctions electrophysiologically. 3. Axons of the sympathetic preganglionic splanchnic nerve (s.p.n.) grow throughout the muscle, but only a small percentage of fibres form synapses. Most e.p.p.s are of low quantal content, generally subthreshold. Long onset latencies and multiple post-synaptic responses indicate that innervation is multiple, multi-terminal, and by unmyelinated axons. 4. Spontaneous miniature e.p.p.s at splanchnic junctions occur at an average rate under 0.1/sec. Their average amplitude and time course are about the same as for control muscles, but the variability of amplitudes is greater than for control muscles. 5. The amount of facilitation shown by s.p.n.-evoked e.p.p.s is the same as by s.m.n. e.p.p.s, but the time course is almost twice as long. 6. S.p.n.-reinnervated fibres show dramatic post-tetanic potentiation preceded by depression, following as few as 20--50 stimuli. 7. As judged by standard physiological and histochemical criteria, AChEsterase is absent at s.p.n. junctions. 8. The pharmacological responses of the s.p.n. junctions are similar to those of normal or foreign s.m.n. innervated neuromuscular junctions in their sensitivity to the cholinergic blocking agents D-tubocurarine and hexamethonium. 9 The s.p.n. is capable of restricting ACh sensitivity to the sites of nerve contacts, although this restriction occurs more slowly and less completely than with s.m.n. reinnervation. The loss of extrajunctional ACh sensitivity can be correlated with effectiveness of innervation; but significant restriction occurs even in s.p.n. reinnervated fibres that probably never contract to nerve stimulation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. On the quantal release of the transmitter at a sympathetic synapse. J Physiol. 1963 Jul;167:402–415. doi: 10.1113/jphysiol.1963.sp007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. O., SATINSKY V. P. Functional restoration of the paralyzed diaphragm following the cross-union of the vagus and phrenic nerves. Am J Med Sci. 1951 Dec;222(6):613–622. doi: 10.1097/00000441-195112000-00001. [DOI] [PubMed] [Google Scholar]
- Beattie J., Duel A. B., Ballance C. The Effects of Stimulation of the Hypothalamic Pupillo-Dilator Centre after Successful Anastomoses Between the Cervical Sympathetic and Certain Motor Nerves. J Anat. 1932 Apr;66(Pt 3):283–299. [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B. M., Gutmann E. Regneration in free grafts of normal and denervated muscles in the rat: morphology and histochemistry. Anat Rec. 1975 Sep;183(1):47–62. doi: 10.1002/ar.1091830106. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- GIACOBINI E. Histochemical demonstration of AChE activity in isolated nerve cells. Acta Physiol Scand. 1956 May 18;36(3):276–290. doi: 10.1111/j.1748-1716.1956.tb01325.x. [DOI] [PubMed] [Google Scholar]
- GUTH L., FRANK K. Restoration of diaphragmatic function following vagophrenic anastomosis in the rat. Exp Neurol. 1959 Apr;1(1):1–12. doi: 10.1016/0014-4886(59)90009-3. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Letinsky M. S., Rheuben M. B. Competitive interaction between foreign nerves innervating frog skeletal muscle. J Physiol. 1979 Apr;289:241–262. doi: 10.1113/jphysiol.1979.sp012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Landmesser L. Contractile and electrical responses of vagus-innervated frog sartorius muscles. J Physiol. 1971 Mar;213(3):707–725. doi: 10.1113/jphysiol.1971.sp009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. Pharmacological properties, cholinesterase activity and anatomy of nerve-muscle junctions in vagus-innervated frog sartorius. J Physiol. 1972 Jan;220(1):243–256. doi: 10.1113/jphysiol.1972.sp009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The union of different kinds of nerve fibres. J Physiol. 1904 Aug 22;31(5):365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- PICK J. The submicroscopic organization of the sympathetic ganglion in the frog (Rana pipiens). J Comp Neurol. 1963 Jun;120:409–462. doi: 10.1002/cne.901200304. [DOI] [PubMed] [Google Scholar]
- Ramirez B., Luco J. V. Some physiological and biochemical features of striated muscles reinnervated by preganglionic sympathetic fibers. J Neurobiol. 1973;4(6):525–533. doi: 10.1002/neu.480040605. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Further analysis of relationship between end-plate potential and end-plate current. J Neurophysiol. 1960 Jul;23:397–402. doi: 10.1152/jn.1960.23.4.397. [DOI] [PubMed] [Google Scholar]
- Vera C. L., Luco J. V. Reinnervation of smooth and striated muscle by sensory nerve fibers. J Neurophysiol. 1967 May;30(3):620–627. doi: 10.1152/jn.1967.30.3.620. [DOI] [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]