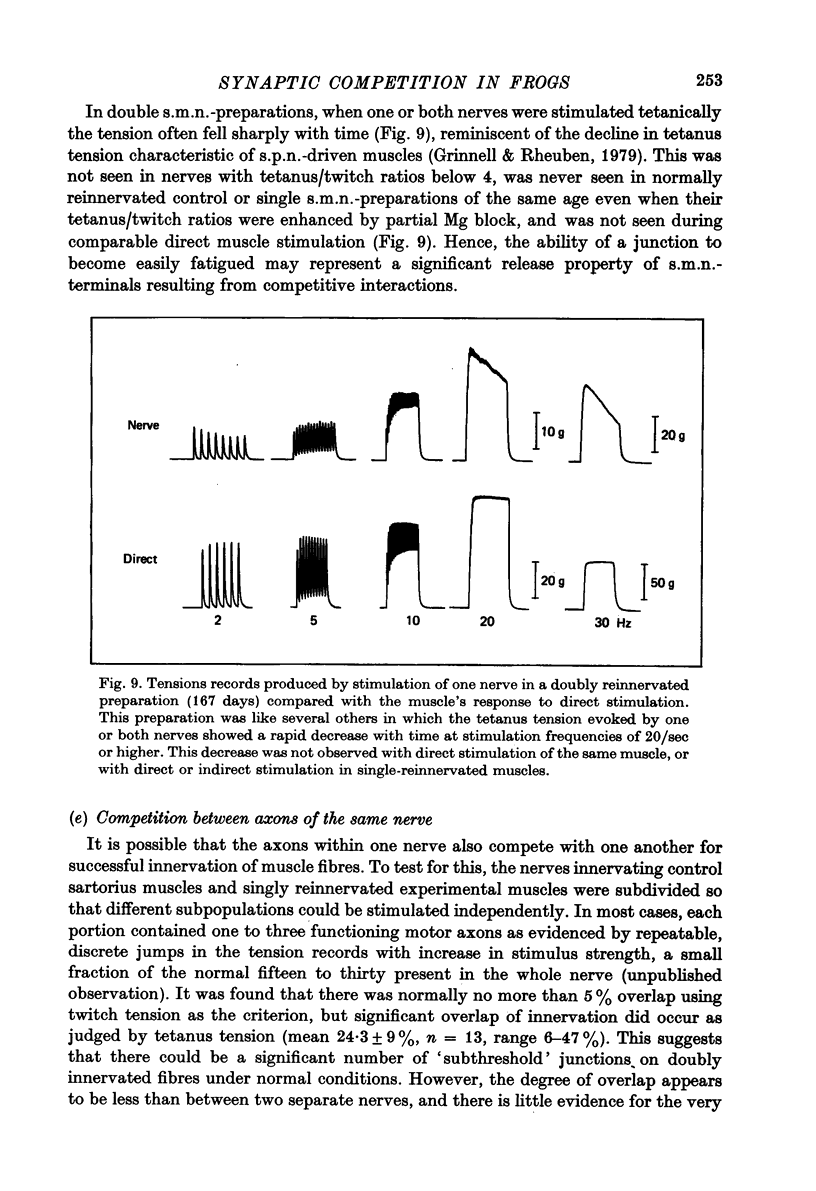
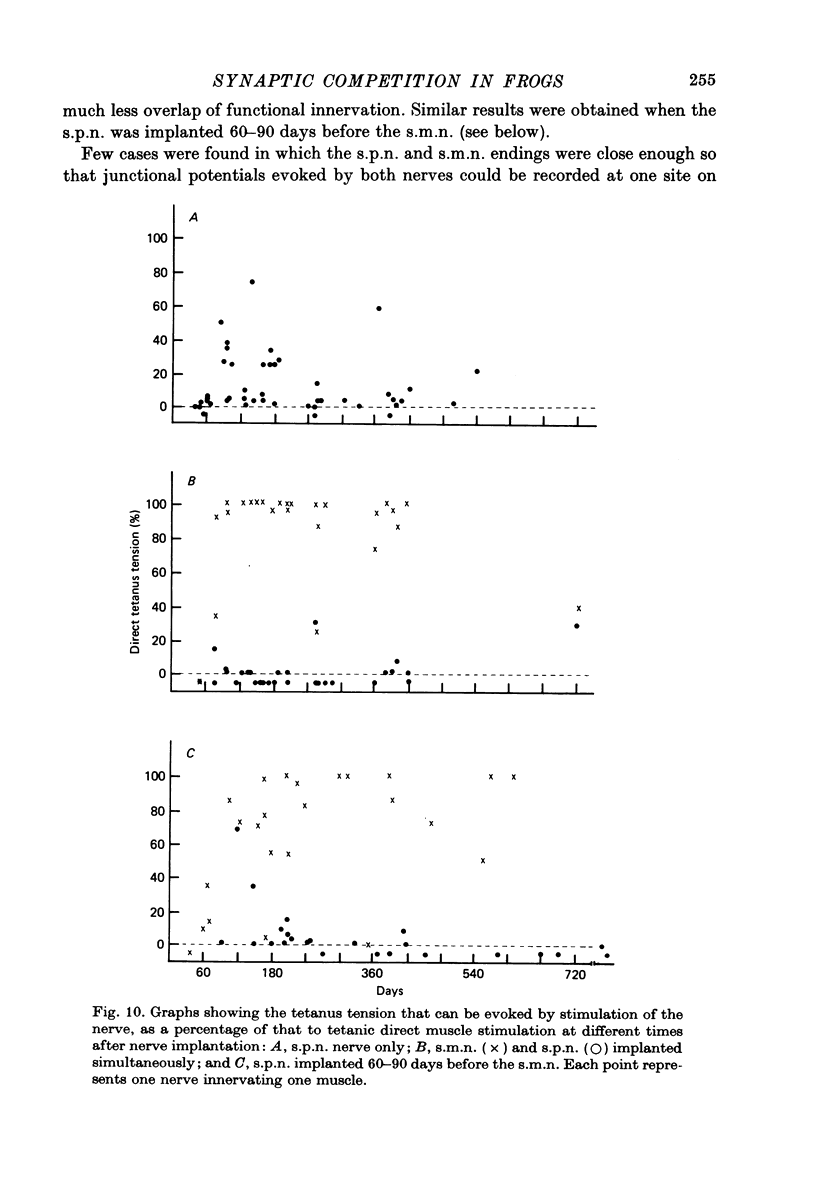
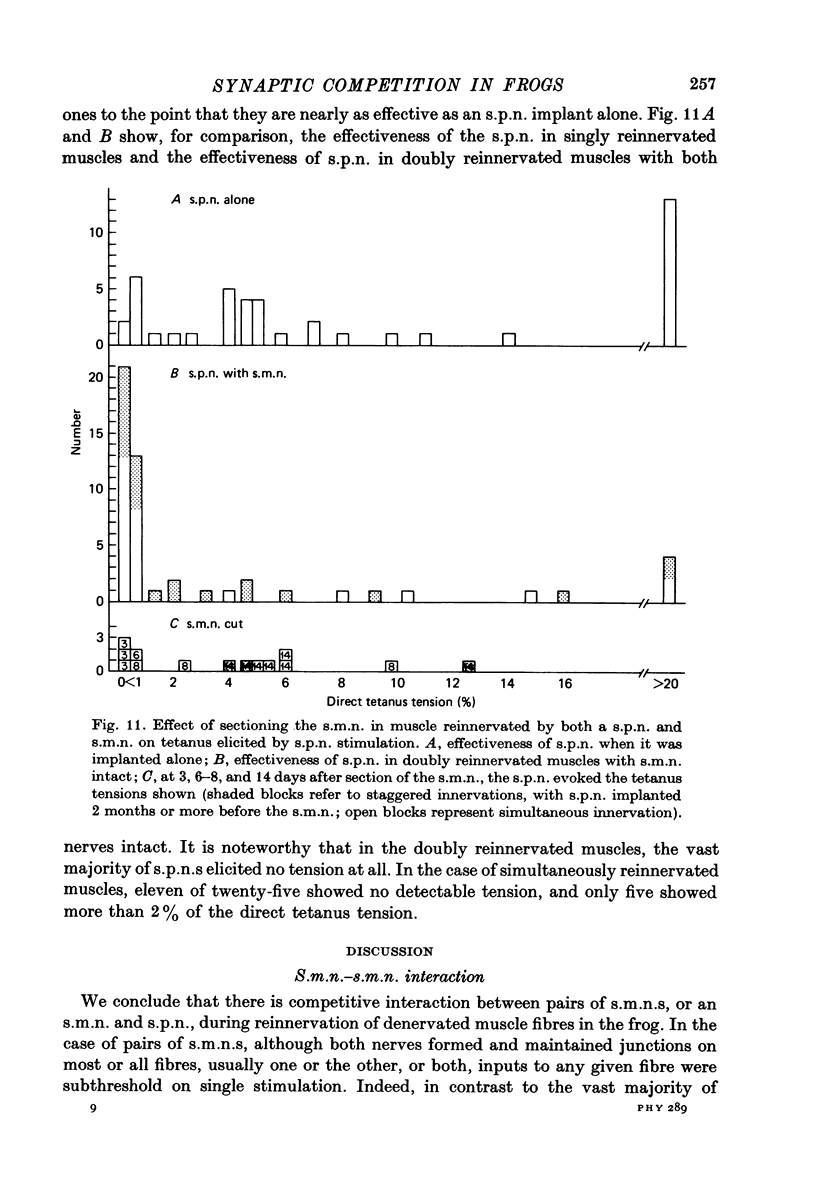
Abstract
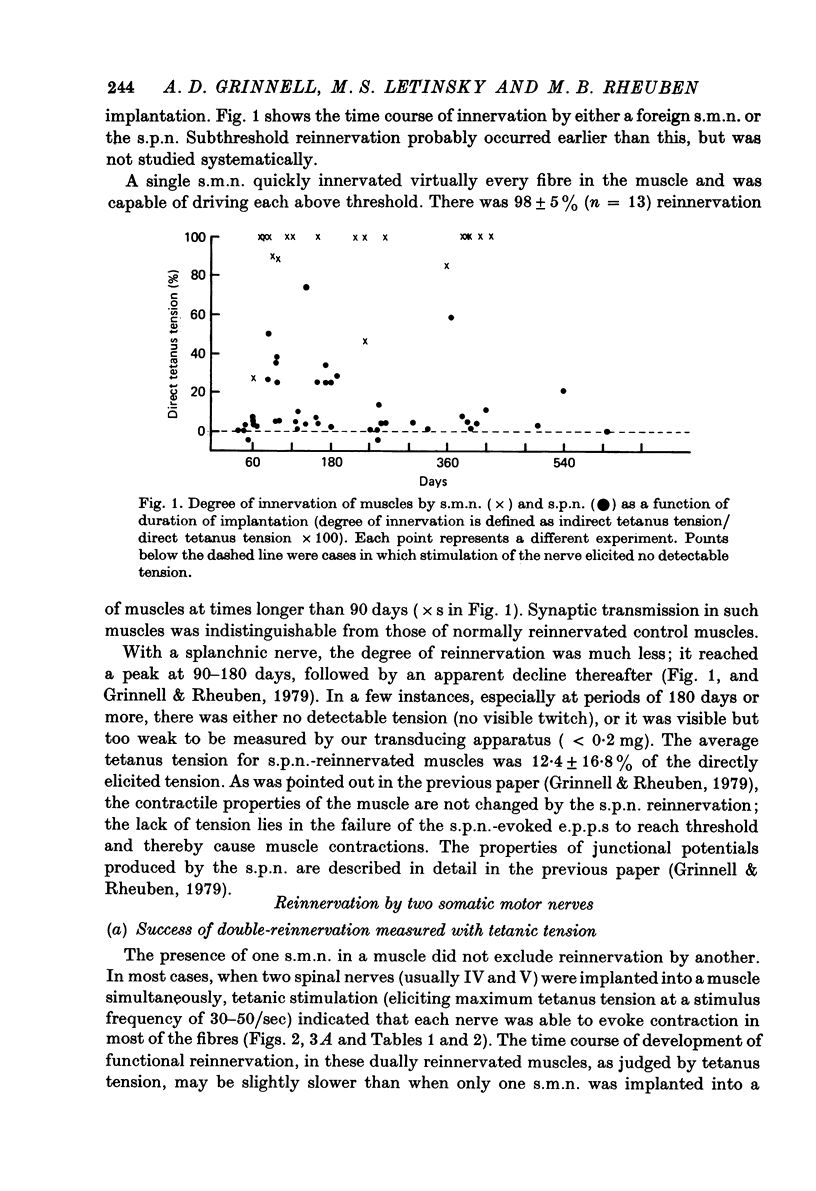
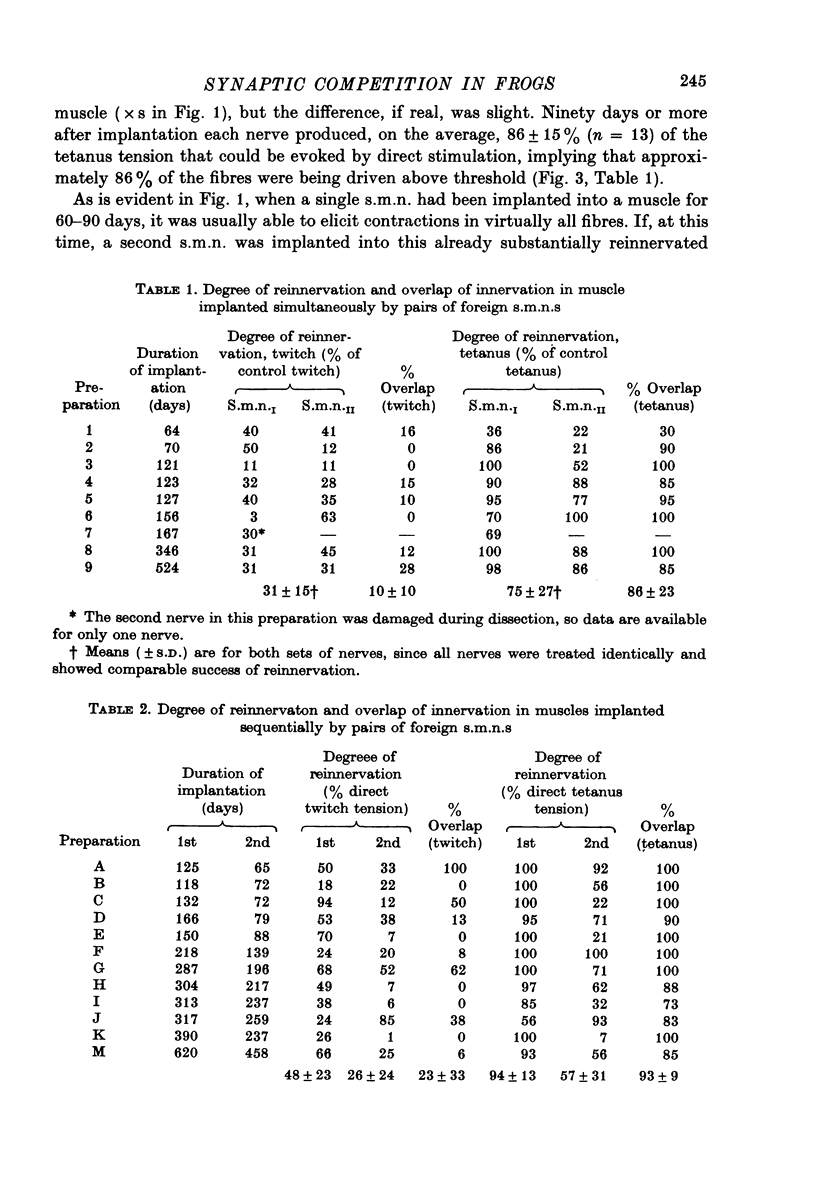
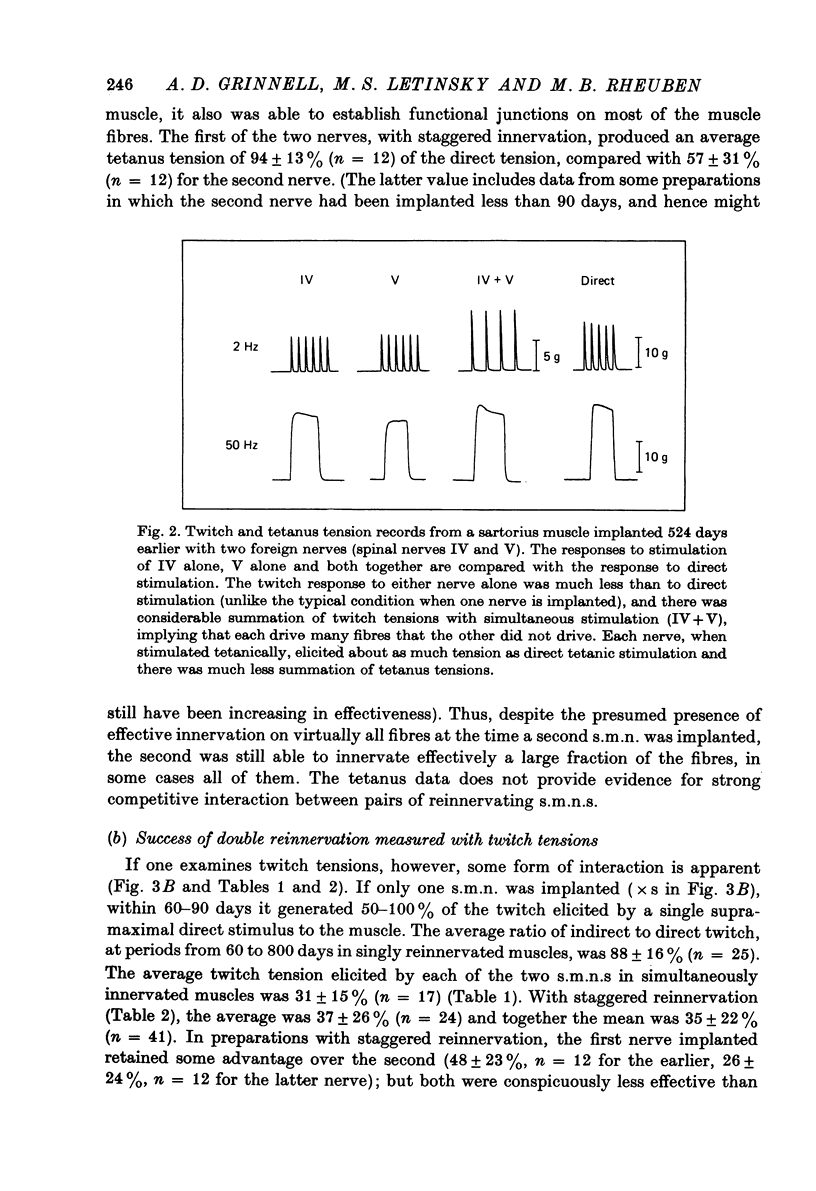
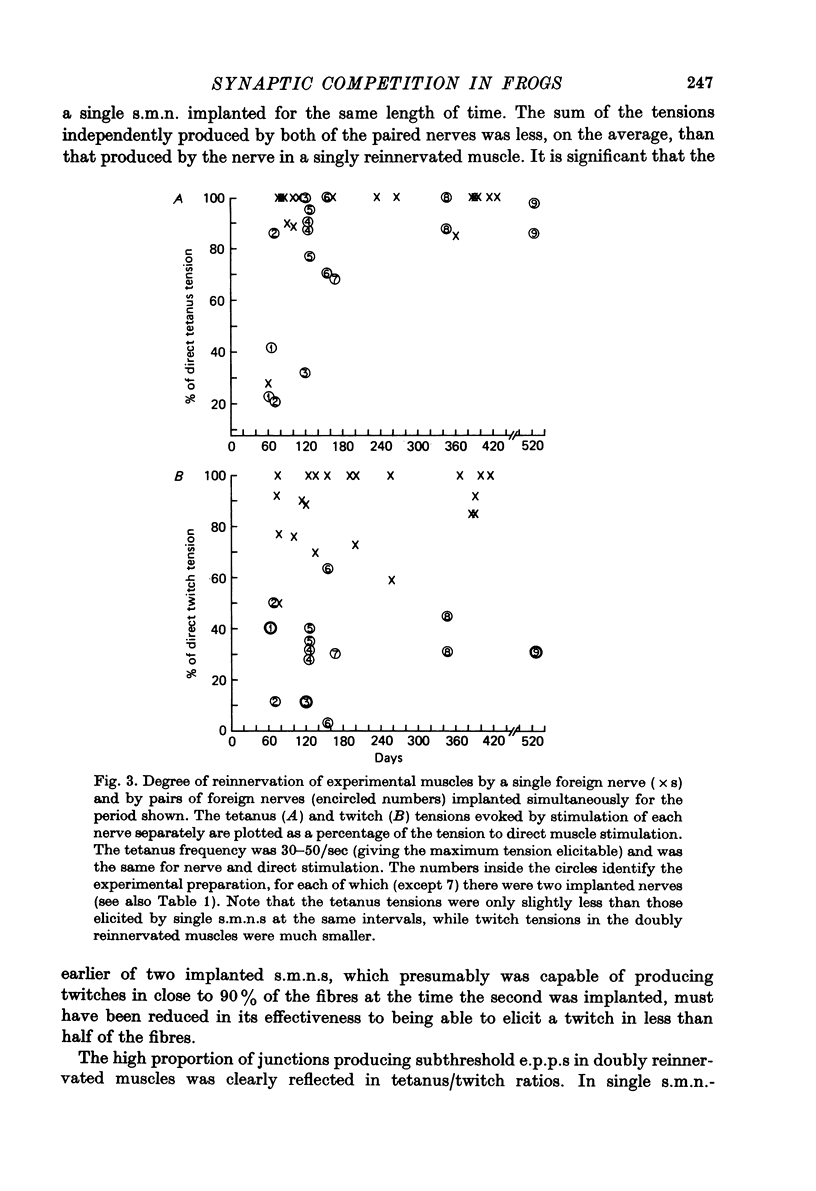
1. Competition between two foreign nerves innervating frog skeletal muscle has been studied by using pairs of somatic motor nerves (s.m.n.s) or one s.m.n. and the preganglionic splanchnic nerve (s.p.n.) implanted into a denervated sartorius muscle that has been transplanted to the lymph sac of the back. 2. A single s.m.n. implanted into the muscle succeeded in innervating essentially every fibre within 2--3 months; tetanic stimulation of the nerve elicited 9--100% of the maximal direct tetanus tension. Most of the e.p.p.s were suprathreshold, since a single indirect stimulus evoked a twitch 60--100% as large as that to a direct stimulus. 3. If two s.m.n.s were implanted simultaneously, tetanic stimulation of either elicited 80--100% of the maximal tension to direct stimulation. If one nerve was implanted 2--3 months before the other, the second, although usually less effective than the first, normally innervated 50--100% of the fibres, with approximately the same time course of innervation as a single s.m.n. 4. Mutual synaptic repression was seen on examination of twitch tensions. With either simultaneous or staggered innervation, stimulation of each s.m.n. resulted in a twitch of 30--50% of the total direct twitch tension, with little overlap between the fields driven by the two nerves. Intracellular recordings showed that the distribution of subthreshold and spike-producing e.p.p.s reflected the existence of separate twitch fields. Even if one s.m.n. was implanted several months before the other and had time to establish suprathreshold junctions on most muscle fibres, an s.m.n. implanted later was able to reduce sharply the effectiveness of many junctions from the earlier nerve while itself innervating most muscle fibres. 5. The subthreshold e.p.p.s had low quantal content, typically ten or fewer quanta/e.p.p. The min e.p.p. frequency was very low, while min e.p.p. amplitude appeared to be normal. 6. In the vast majority of muscle fibres, junctions from the two nerves were not within recording distance of each other. Hence, we infer that the competitive interaction was mediated somehow via the muscle fibre. 7. The preganglionic splanchnic nerve, which also successfully reinnervated frog skeletal muscle, competed with a foreign s.m.n. in ways which differ qualitatively from the competition by a second s.m.n. In the presence of a s.m.n., synapses of the s.p.n. were almost universally subthreshold. However, if the s.p.n. was implanted 2--3 months before the s.m.n., the s.m.n. was prevented for several months from innervating fibres driven by the s.p.n. This delay in s.m.n. reinnervation was greater than if the first nerve implanted was also an s.m.n. 8. After 6--8 months of dual innervation by s.m.n. and s.p.n., the s.m.n. became almost totally dominant. However, if the s.m.n. was then sectioned, the s.p.n. became as effective, within approximately 1 week, as it would have been in the absence of the s.m.n.
Full text
PDF



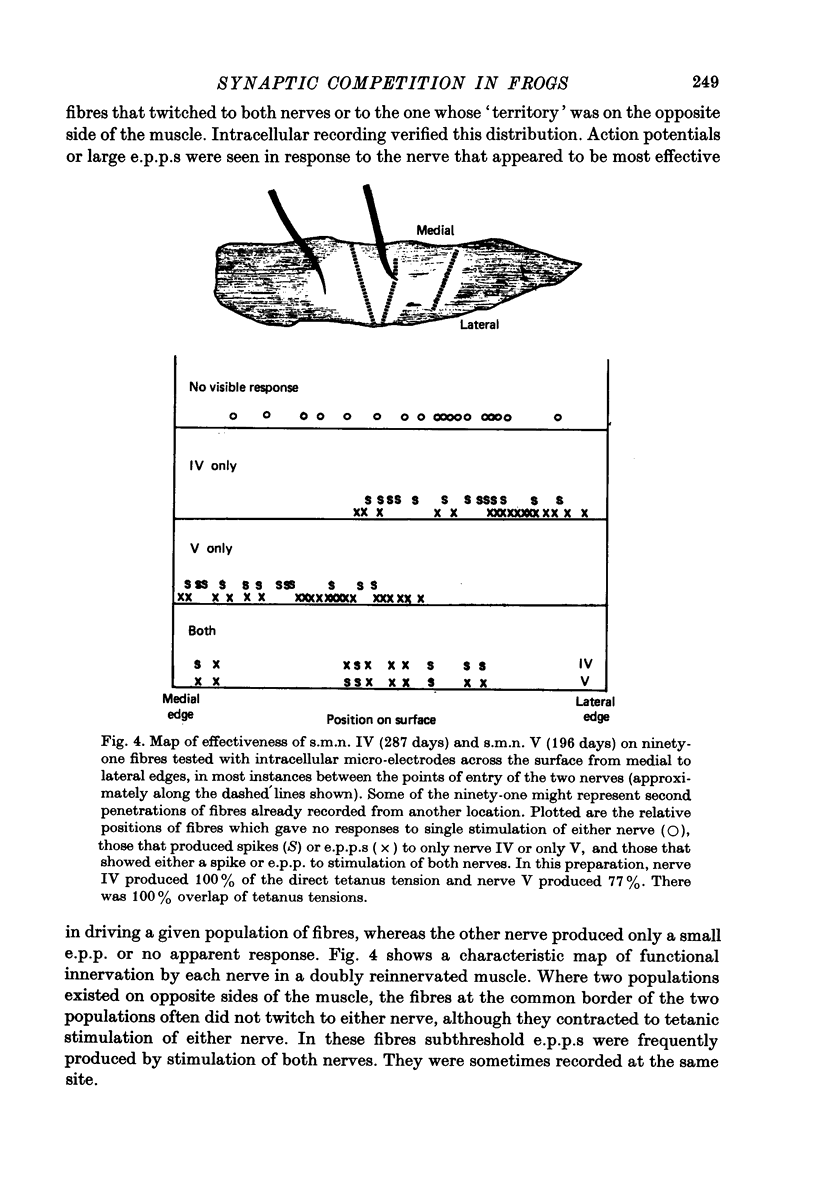
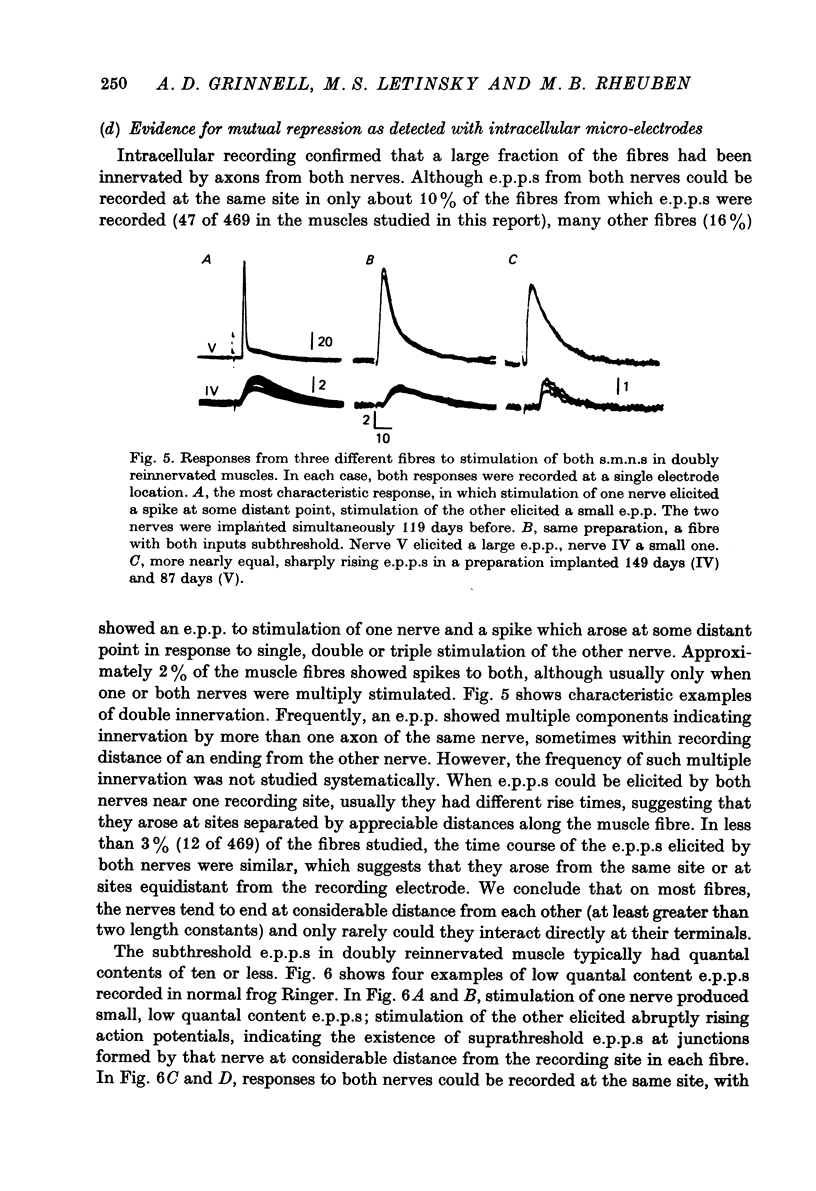
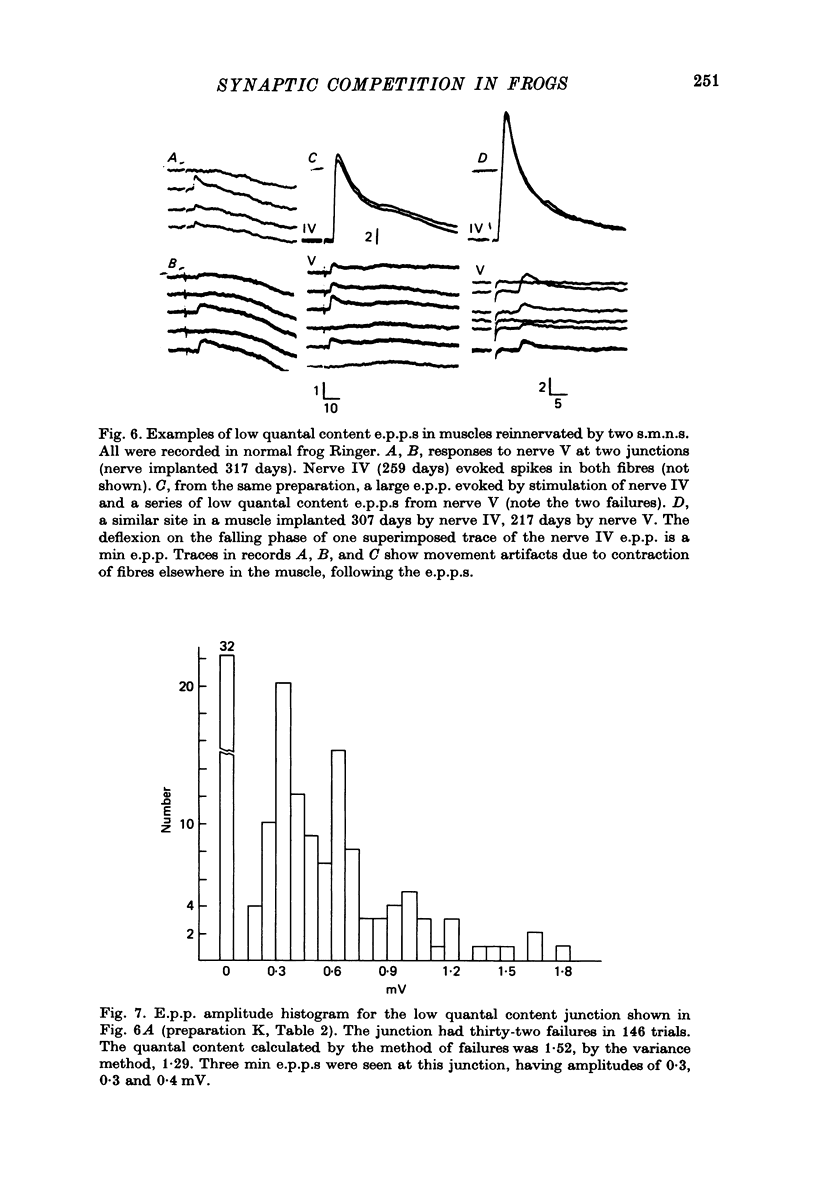
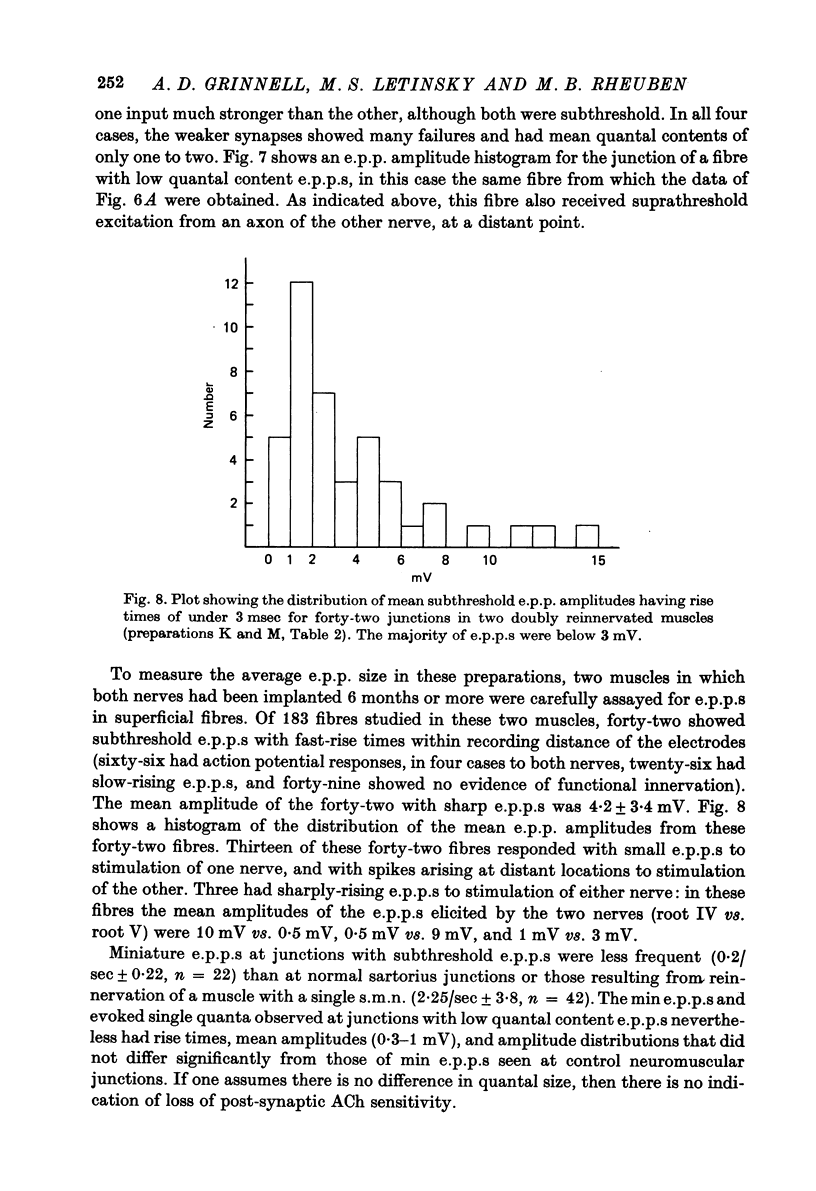







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. T. Growth of nerve implants in voluntary muscle. J Anat. 1950 Jan;84(Pt 1):38–49. [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN J. J., GUTH L. Nonselectivity in establishment of neuromuscular connections following nerve regeneration in the rat. Exp Neurol. 1961 Sep;4:262–275. doi: 10.1016/0014-4886(61)90047-4. [DOI] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass D. T., Sutton T. J., Mark R. F. Competition between nerves for functional connexions with axolotl muscles. Nature. 1973 May 25;243(5404):201–203. doi: 10.1038/243201a0. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Yip J. W. Formation and elimination of foreign synapses on adult salamander muscle. J Physiol. 1978 Jan;274:299–310. doi: 10.1113/jphysiol.1978.sp012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul R., Miledi R., Stefani E. Neural control of contracture in slow muscle fibres of the frog. Acta Physiol Lat Am. 1970;20(3):194–226. [PubMed] [Google Scholar]
- Frank E., Jansen J. K. Interaction between foreign and original nerves innervating gill muscles in fish. J Neurophysiol. 1976 Jan;39(1):84–90. doi: 10.1152/jn.1976.39.1.84. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. Maintained function of foreign synapses on hyperinnervated skeletal muscle fibres of the rat. Nature. 1974 Feb 8;247(5440):375–376. doi: 10.1038/247375a0. [DOI] [PubMed] [Google Scholar]
- GUTH L. Neuromuscular function after regeneration of interrupted nerve fibers into partially denervated muscle. Exp Neurol. 1962 Aug;6:129–141. doi: 10.1016/0014-4886(62)90083-3. [DOI] [PubMed] [Google Scholar]
- Grimm L. M. An evaluation of myotypic respecification in axolotls. J Exp Zool. 1971 Dec;178(4):479–496. doi: 10.1002/jez.1401780406. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B., Letinsky M. S. Repression of synaptic efficacy in frog skeletal muscle. Nature. 1977 Jun 30;267(5614):866–866. doi: 10.1038/267866b0. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B., Letinsky M. S. Repression of synaptic efficacy in frog skeletal muscle. Nature. 1977 Jun 30;267(5614):866–866. doi: 10.1038/267866b0. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B. The physiology, pharmacology, and trophic effectiveness of synapses formed by autonomic preganglionic nerves on frog skeletal muscle. J Physiol. 1979 Apr;289:219–240. doi: 10.1113/jphysiol.1979.sp012734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V. Effects of accessory nerve supply to muscle achieved by implantation into muscle during regeneration of its nerve. Physiol Bohemoslov. 1967;16(3):244–250. [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Motor innervation of skeletal muscle: multiple innervation of individual muscle fibres and motor unit function. J Physiol. 1954 Nov 29;126(2):293–303. doi: 10.1113/jphysiol.1954.sp005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hník P., Jirmanová I., Vyklický L., Zelená J. Fast and slow muscles of the chick after nerve cross-union. J Physiol. 1967 Nov;193(2):309–325. doi: 10.1113/jphysiol.1967.sp008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWASAKI S. Mononeuronal double innervation of an amphibian striated muscle fibre. Jpn J Physiol. 1957 Dec 20;7(4):267–275. doi: 10.2170/jjphysiol.7.267. [DOI] [PubMed] [Google Scholar]
- Landmesser L. Contractile and electrical responses of vagus-innervated frog sartorius muscles. J Physiol. 1971 Mar;213(3):707–725. doi: 10.1113/jphysiol.1971.sp009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R., Mart P. E. The mechanism of selective reinnervation of fish eye muscles. IV. Identification of repressed synapses. Brain Res. 1972 Nov 13;46:149–157. doi: 10.1016/0006-8993(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R. The mechanism of selective reinnervation of fish eye muscles. 3. Functional, electrophysiological and anatomical analysis of recovery from section of 3rd and IVth nerves. Brain Res. 1972 Nov 13;46:131–148. doi: 10.1016/0006-8993(72)90011-x. [DOI] [PubMed] [Google Scholar]
- Marotte L. R., Mark R. F. The mechanism of selective reinnervation of fish eye muscle. I. Evidence from muscle function during recovery. Brain Res. 1970 Apr 1;19(1):41–51. doi: 10.1016/0006-8993(70)90235-0. [DOI] [PubMed] [Google Scholar]
- Marotte L. R., Mark R. P. The mechanism of selective reinnervation of fish eye muscle. II. Evidence from electronmicroscopy of nerve endings. Brain Res. 1970 Apr 1;19(1):53–62. doi: 10.1016/0006-8993(70)90236-2. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Steinbach A. B. Induction of the action potential mechanism in slow muscle fibres of the frog. J Physiol. 1971 Sep;217(3):737–754. doi: 10.1113/jphysiol.1971.sp009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Competitive and non-competitive re-innervation of mammalian sympathetic neurones by native and foreign fibres. J Physiol. 1976 Oct;261(2):453–475. doi: 10.1113/jphysiol.1976.sp011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. L., Taraskevich P. S. Reduction of multiaxonal innervation at the neuromuscular junction of the rat during development. J Physiol. 1977 Sep;270(2):299–310. doi: 10.1113/jphysiol.1977.sp011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. A. Maintained function of foreign and appropriate junctions on reinnervated goldfish extraocular muscles. J Physiol. 1977 Jun;268(1):87–109. doi: 10.1113/jphysiol.1977.sp011848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry R. W., Arora H. L. Selectivity in regeneration of the oculomotor nerve in the cichlid fish, Astronotus ocellatus. J Embryol Exp Morphol. 1965 Dec;14(3):307–317. [PubMed] [Google Scholar]
- Stefani E., Schmidt H. Early stage of re-innervation of frog slow muscle fibres. Pflugers Arch. 1972;336(3):271–275. doi: 10.1007/BF00590051. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Magazanik L. G. Dual end-plate potentials at the single neuromuscular junction of the adult frog. Pflugers Arch. 1977 Apr 25;368(3):271–273. doi: 10.1007/BF00585207. [DOI] [PubMed] [Google Scholar]
- Yip J. W., Dennis M. J. Suppression of transmission at foreign synapses in adult newt muscle involves reduction in quantal content. Nature. 1976 Mar 25;260(5549):350–352. doi: 10.1038/260350a0. [DOI] [PubMed] [Google Scholar]


