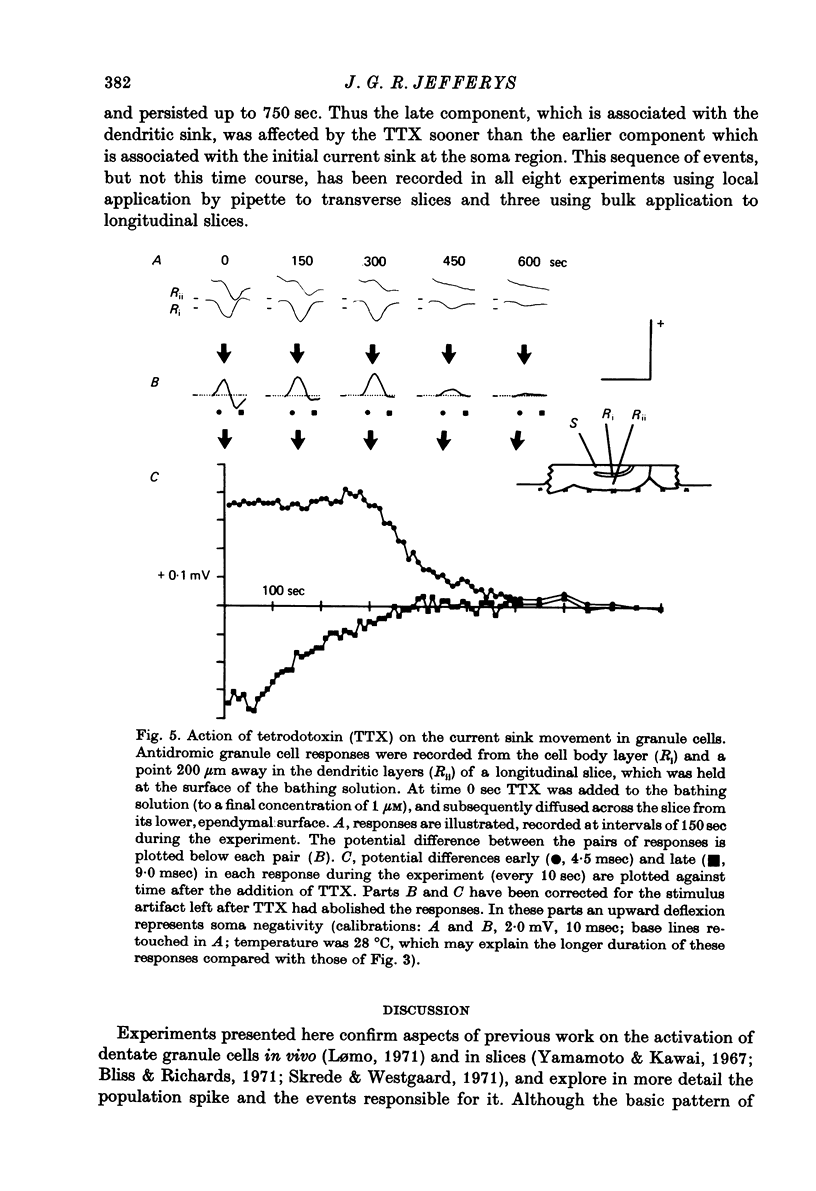
Abstract
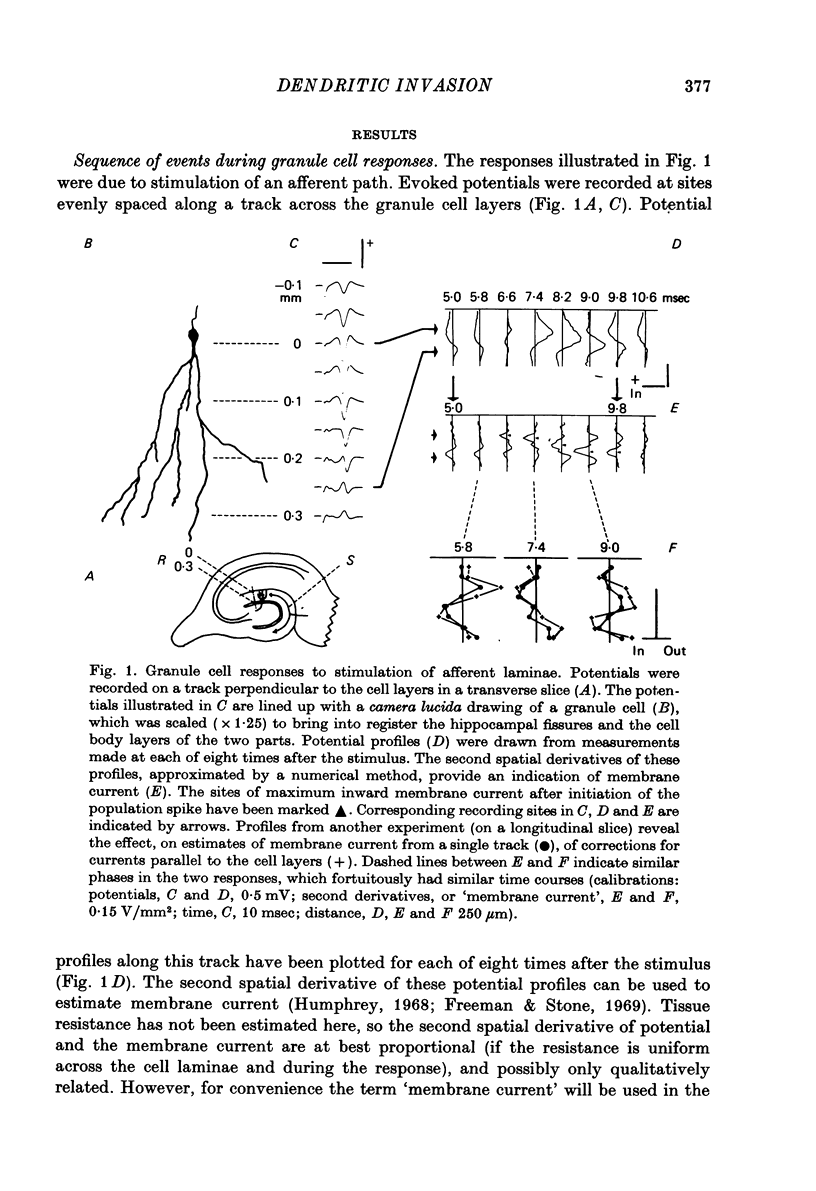
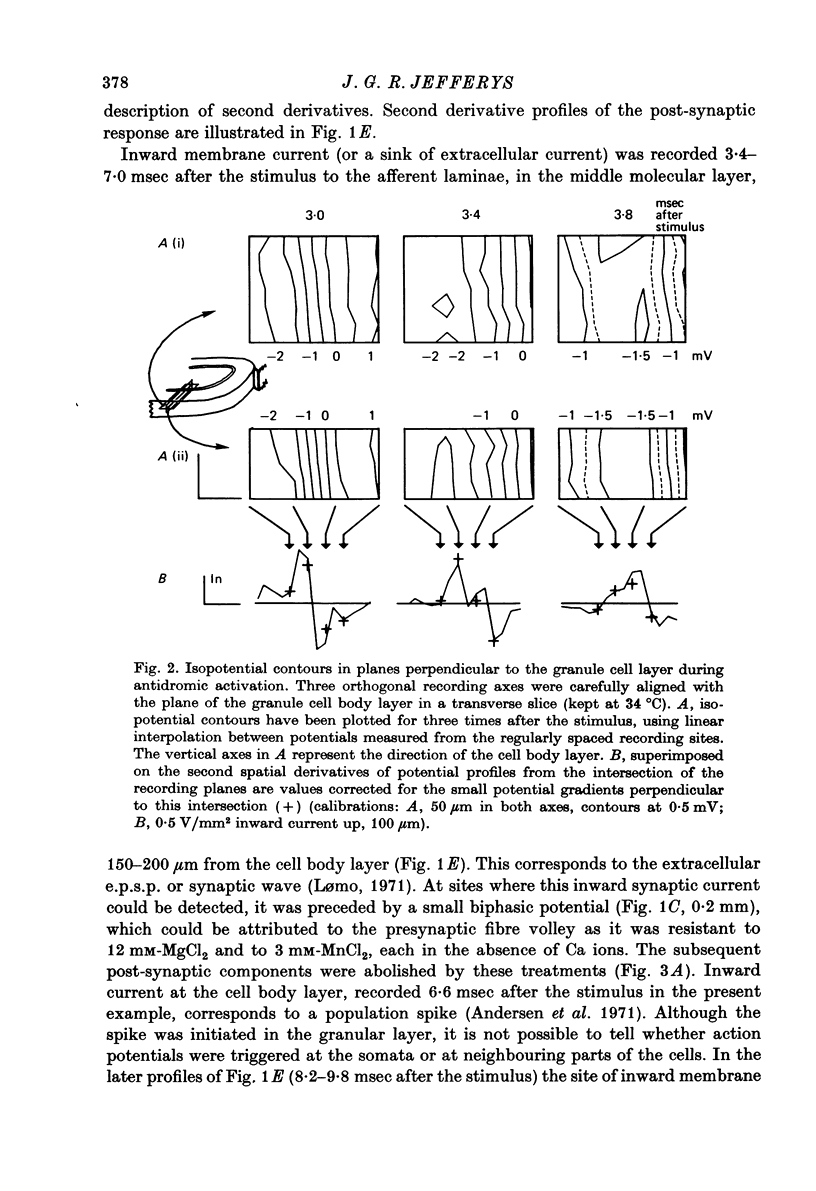
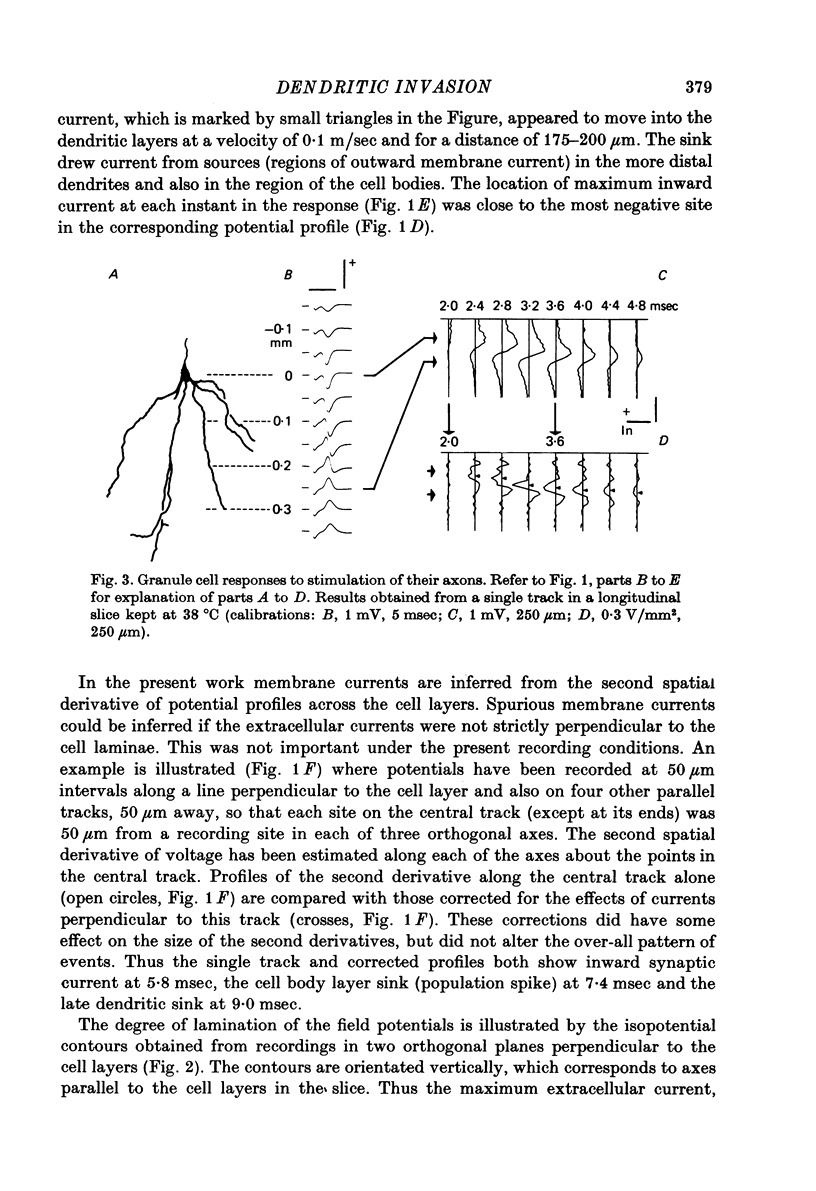
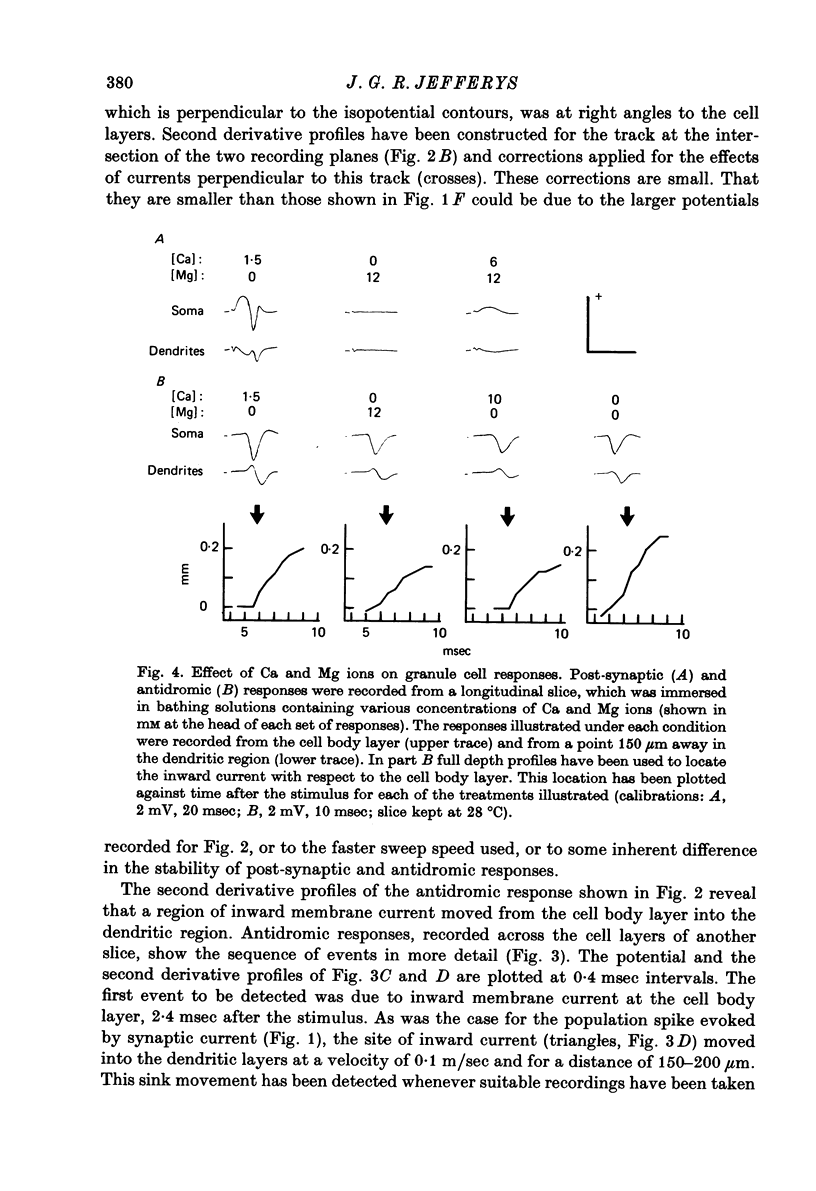
1. Laminar field potentials due to the synchronous activation of granule cells were studied in slices of guinea-pig hippocampus maintained in vitro. 2. Extracellular recordings confirmed that stimulation of afferent laminae in the molecular layer caused excitatory synaptic current to enter the granule cell dendrites. If large enough this current initiated action potentials at, or near to, the somata 100--200 micrometers away. 3. After a population spike had been initiated via excitatory synapses or via antidromic invasion, the lcoation of inward membrane current (sink) appeared to move from the cell body layer into the dendrites at a velocity of 0.08-0.12 m/sec, for a distance of up to 250 micrometers. 4. The sink movement into the dendrites was blocked by tetrodotoxin and not by agents that blocked synaptic activation. Together with other observations these results led to the conclusion that granule cell dendrites were invaded by action potentials from the cell body region. There was no evidence of dendritic action potentials from the cell body region. There was no evidence of dendritic action potentials preceding the cell body spike initiated by synaptic inputs. Possible functions of this dendritic invasion are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Richards C. D. Some experiments with in vitro hippocampal slices. J Physiol. 1971;214 (Suppl):7P–9P. [PubMed] [Google Scholar]
- Calvin W. H., Hellerstein D. Dendritic spikes versus cable properties. Science. 1969 Jan 3;163(3862):96–97. doi: 10.1126/science.163.3862.96. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler S. A., Dudek F. E., Cotman C. W., Lynch G. Intracellular responses of rat dentate granule cells in vitro: posttetanic potentiation to perforant path stimulation. Brain Res. 1975 Apr 25;88(1):80–85. doi: 10.1016/0006-8993(75)90951-8. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Deadwyler S. A., Cotman C. W., Lynch G. Intracellular responses from granule cell layer in slices of rat hippocampus: perforant path synapse. J Neurophysiol. 1976 Mar;39(2):384–393. doi: 10.1152/jn.1976.39.2.384. [DOI] [PubMed] [Google Scholar]
- FATT P. Electric potentials occurring around a neurone during its antidromic activation. J Neurophysiol. 1957 Jan;20(1):27–60. doi: 10.1152/jn.1957.20.1.27. [DOI] [PubMed] [Google Scholar]
- FATT P. Sequence of events in synaptic activation of a motoneurone. J Neurophysiol. 1957 Jan;20(1):61–80. doi: 10.1152/jn.1957.20.1.61. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Gardner-Medwin A. R. The effect of synaptic activation on the extracellular potassium concentration in the hippocampal dentate area, in vitro. Brain Res. 1976 Aug 6;112(1):183–187. doi: 10.1016/0006-8993(76)90348-6. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R. Re-analysis of the antidromic cortical response. I. Potentials evoked by stimulation of the isolated pyramidal tract. Electroencephalogr Clin Neurophysiol. 1968 Feb;24(2):116–129. doi: 10.1016/0013-4694(68)90118-1. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Proceedings: Propagation of action potentials into the dendrites of hippocampal granule cells in vitro. J Physiol. 1975 Jul;249(1):16P–18P. [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Katz B. The effect of electrolyte deficiency on the rate of conduction in a single nerve fibre. J Physiol. 1947 Oct 15;106(4):411–417. doi: 10.1113/jphysiol.1947.sp004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C., Freeman J. A., Hillman D. E. Dendritic spikes and their inhibition in alligator Purkinje cells. Science. 1968 Jun 7;160(3832):1132–1135. doi: 10.1126/science.160.3832.1132. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- NELSON P. G., FRANK K. EXTRACELLULAR POTENTIAL FIELDS OF SINGLE SPINAL MOTONEURONS. J Neurophysiol. 1964 Sep;27:913–927. doi: 10.1152/jn.1964.27.5.913. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Freeman J. A. Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol. 1975 Mar;38(2):356–368. doi: 10.1152/jn.1975.38.2.356. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Llinas R. Field potentials in the alligator cerebellum and theory of their relationship to Purkinje cell dendritic spikes. J Neurophysiol. 1971 Jul;34(4):509–531. doi: 10.1152/jn.1971.34.4.509. [DOI] [PubMed] [Google Scholar]
- RANCK J. B., Jr Specific impedance of rabbit cerebral cortex. Exp Neurol. 1963 Feb;7:144–152. doi: 10.1016/s0014-4886(63)80005-9. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Westgaard R. H. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971 Dec 24;35(2):589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- TERZUOLO C. A., ARAKI T. An analysis of intra- versus extracellular potential changes associated with activity of single spinal motoneurons. Ann N Y Acad Sci. 1961 Sep 6;94:547–558. doi: 10.1111/j.1749-6632.1961.tb35558.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Kawai N. Presynaptic action of acetylcholine in thin sections from the guinea pig dentate gyrus in vitro. Exp Neurol. 1967 Oct;19(2):176–187. doi: 10.1016/0014-4886(67)90016-7. [DOI] [PubMed] [Google Scholar]
- Yedlin M., Kwan H., Murphy J. T., Nguyen-Huu H., Wong Y. C. Electrical conductivity in cat cerebellar cortex. Exp Neurol. 1974 Jun;43(3):555–569. doi: 10.1016/0014-4886(74)90195-2. [DOI] [PubMed] [Google Scholar]


