Abstract
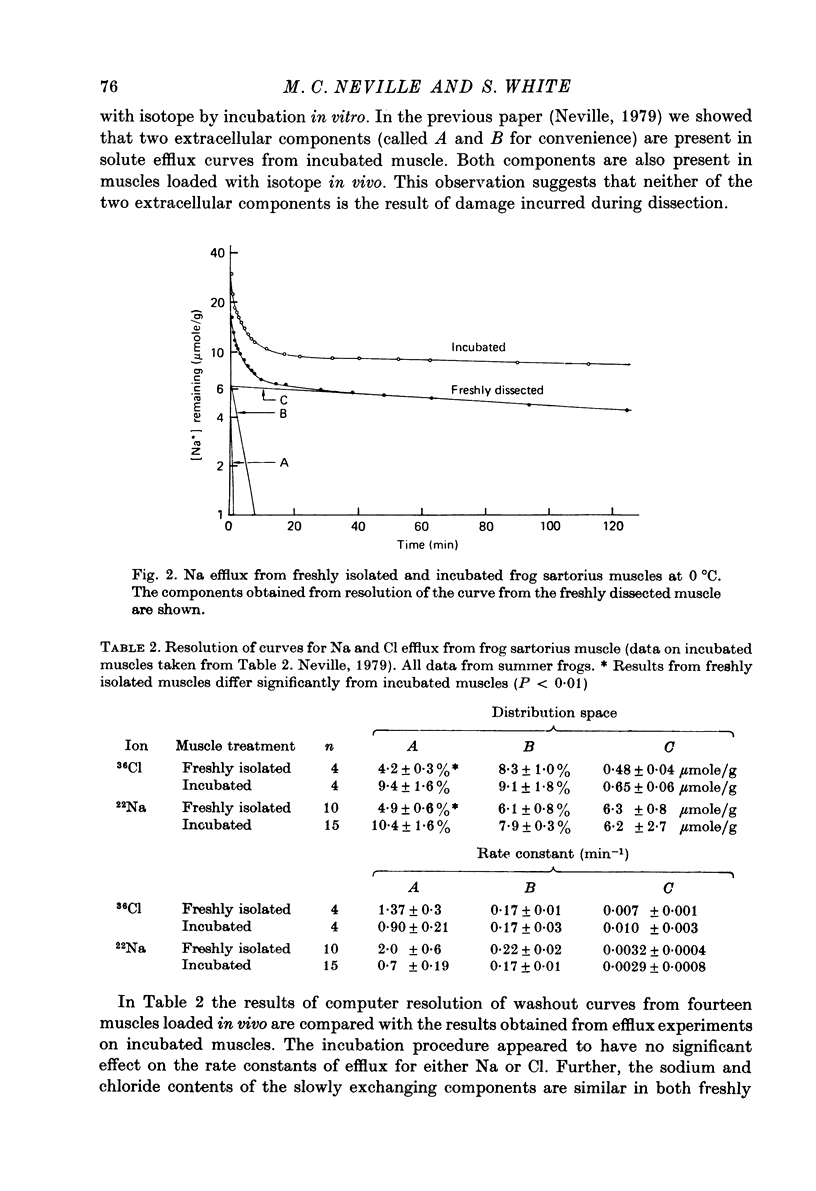
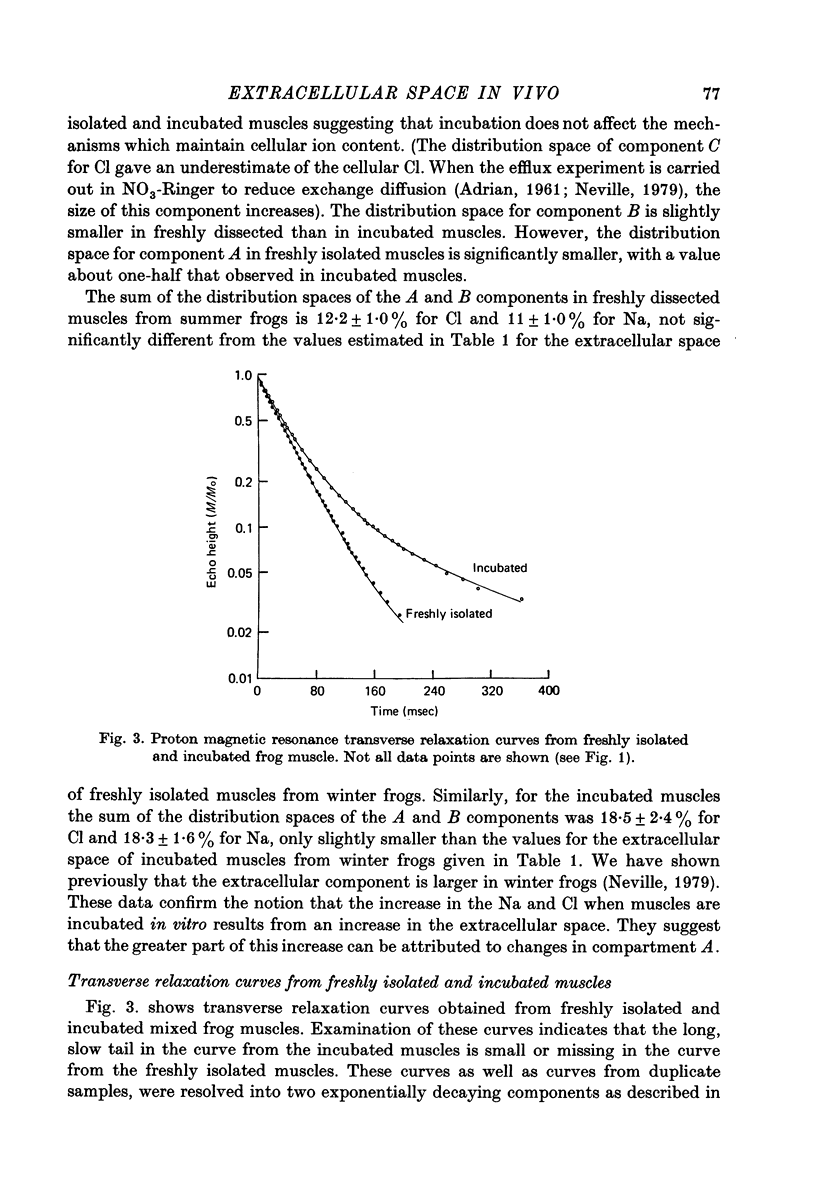
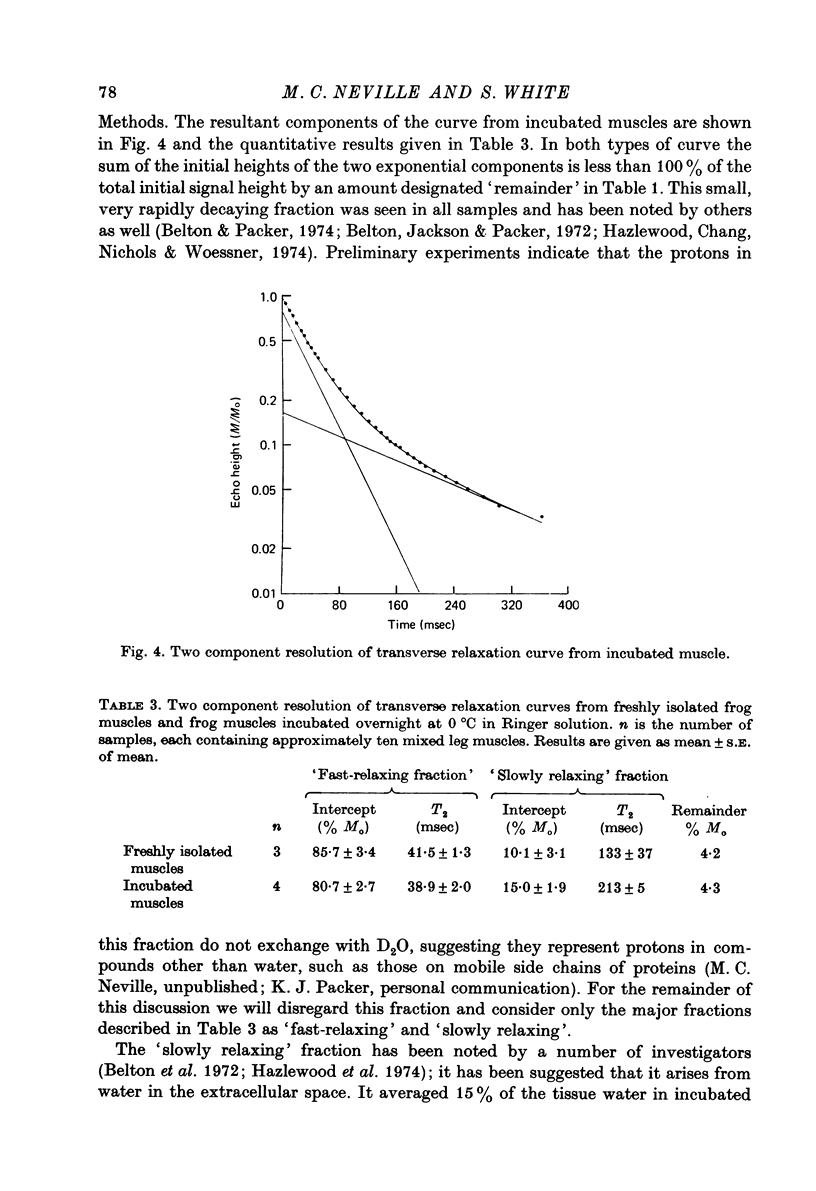
1. The Na and Cl distribution spaces of freshly isolated frog muscles are 16.7 and 12.6%, respectively. These values increase to 25.6 and 23.3%, respectively, on incubation. 2. The extracellular components of both Na and Cl efflux curves are significantly smaller in freshly isolated muscles (approximately 12%) than in incubated muscles (approximately 18%). The fast exchanging A component of the extracellular space is increased more by incubation than the more slowly exchanging B component. 3. The proton magnetic resonance (p.m.r.) transverse relaxation curve for the water of freshly isolated frog muscles did not show the long, slowly relaxing tail present in curves from muscles incubated in Ringer solution. 4. When muscles were incubated in hypertonic solutions the p.m.r. transverse relaxation curves could be resolved into three components whose sizes were consistent with the components present in the sodium and chloride efflux curves. The non-exponentiality of the p.m.r. transverse relaxation curve therfore appears to arise from water in both the A and B extracellular compartments of muscle. 5. Efflux analysis indicated that the cellular Na content of both freshly isolated and incubated frog muscle is similar to that predicted by others (Lev, 1964; Armstrong & Lee, 1971; Lee & Armstrong, 1974) from measurements of intracellular Na ion activity using Na-sensitive micro-electrodes. The remainder of the tissue Na was found in the more rapidly exchanging extracellular compartments. The results of these experiments are inconsistent with the presence of a substantial fraction of bound Na in frog muscle. 6. These experiments show that muscle extracellular space is smaller in vivo than in vitro. Efflux analysis is suggested as the most accurate method of assessing extra-cellular components.
Full text
PDF



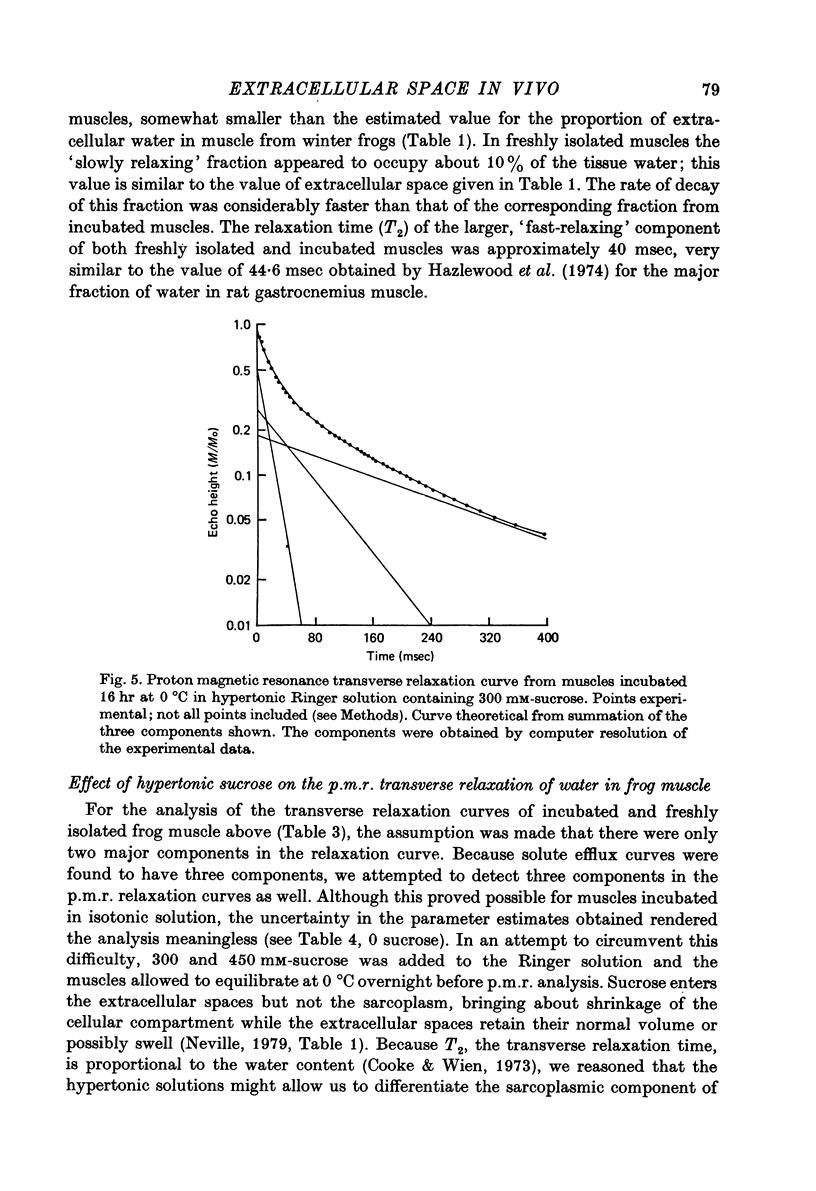



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Internal chloride concentration and chloride efflux of frog muscle. J Physiol. 1961 May;156:623–632. doi: 10.1113/jphysiol.1961.sp006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. M., Lee C. O. Sodium and potassium activities in normal and "sodium-rich" frog skeletal muscle. Science. 1971 Jan 29;171(3969):413–415. doi: 10.1126/science.171.3969.413. [DOI] [PubMed] [Google Scholar]
- Belton P. S., Jackson R. R., Packer K. J. Pulsed NMR studies of water in striated muscle. I. Transverse nuclear spin relaxation times and freezing effects. Biochim Biophys Acta. 1972 Nov 24;286(1):16–25. doi: 10.1016/0304-4165(72)90084-0. [DOI] [PubMed] [Google Scholar]
- Berendsen H. J., Edzes H. T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann N Y Acad Sci. 1973 Mar 30;204:459–485. doi: 10.1111/j.1749-6632.1973.tb30799.x. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J., Kane F., O'reilly H. L. Volume of interfibre spaces in frog muscle and the calculation of concentrations in the fibre water. J Physiol. 1941 Jun 30;99(4):401–414. doi: 10.1113/jphysiol.1941.sp003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Factors governing movement and distribution of inorganic ions in nerve and muscle. Physiol Rev. 1968 Jan;48(1):1–64. doi: 10.1152/physrev.1968.48.1.1. [DOI] [PubMed] [Google Scholar]
- Cooke R., Wien R. Nuclear magnetic resonance studies of intracellular water protons. Ann N Y Acad Sci. 1973 Mar 30;204:197–209. doi: 10.1111/j.1749-6632.1973.tb30780.x. [DOI] [PubMed] [Google Scholar]
- Cope F. W. NMR evidence for complexing of Na+ in muscle, kidney, and brain, and by actomyosin. The relation of cellular complexing of Na+ to water structure and to transport kinetics. J Gen Physiol. 1967 May;50(5):1353–1375. doi: 10.1085/jgp.50.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegel J. G., Pintar M. M. Origin of the nonexponentiality of the water proton spin relaxations in tissues. Biophys J. 1975 Sep;15(9):855–860. doi: 10.1016/S0006-3495(75)85861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton A. C., Coleman T. G. Regulation on interstitial fluid volume and pressure. Ann N Y Acad Sci. 1968 Aug 14;150(3):537–547. doi: 10.1111/j.1749-6632.1968.tb14705.x. [DOI] [PubMed] [Google Scholar]
- Hazlewood C. F., Chang D. C., Nichols B. L., Woessner D. E. Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys J. 1974 Aug;14(8):583–606. doi: 10.1016/S0006-3495(74)85937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEV A. A. DETERMINATION OF ACTIVITY AND ACTIVITY COEFFICIENTS OF POTASSIUM AND SODIUM IONS IN FROG MUSCLE FIBRES. Nature. 1964 Mar 14;201:1132–1134. doi: 10.1038/2011132a0. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. State and distribution of potassium and sodium ions in frog skeletal muscle. J Membr Biol. 1974;15(4):331–362. doi: 10.1007/BF01870094. [DOI] [PubMed] [Google Scholar]
- Macchia D. D., Polimeni P. I., Page E. Cellular Cl content and concentration of amphibian skeletal and heart muscle. Am J Physiol. 1978 Sep;235(3):C122–C127. doi: 10.1152/ajpcell.1978.235.3.C122. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Sodium and water binding in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1966 Sep;44(5):837–848. doi: 10.1139/y66-102. [DOI] [PubMed] [Google Scholar]
- Moller M., Mollgård K., Lund-Andersen H., Hertz L. Concordance between morphological and biochemical estimates of fluid spaces in rat brain cortex slices. Exp Brain Res. 1974;21(3):299–314. doi: 10.1007/BF00235749. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Mathias R. T. The extracellular compartments of frog skeletal muscle. J Physiol. 1979 Mar;288:45–70. [PMC free article] [PubMed] [Google Scholar]


