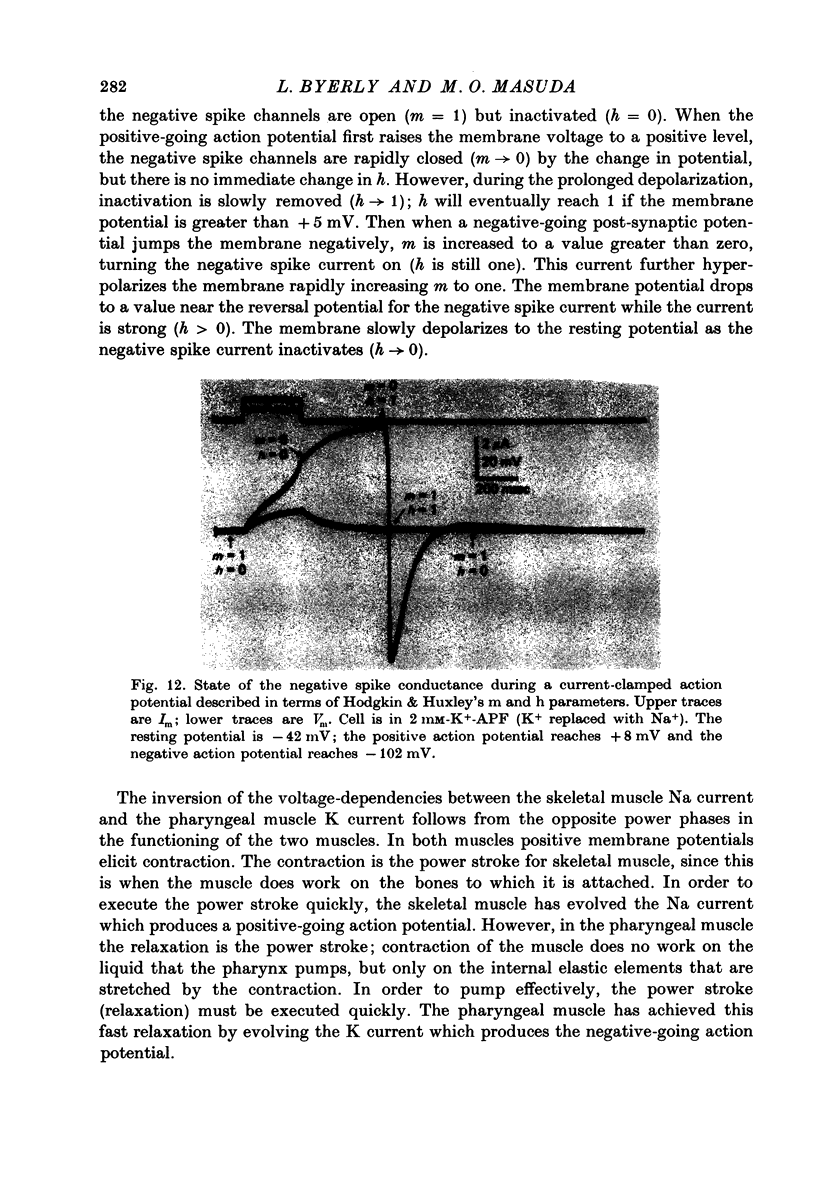
Abstract
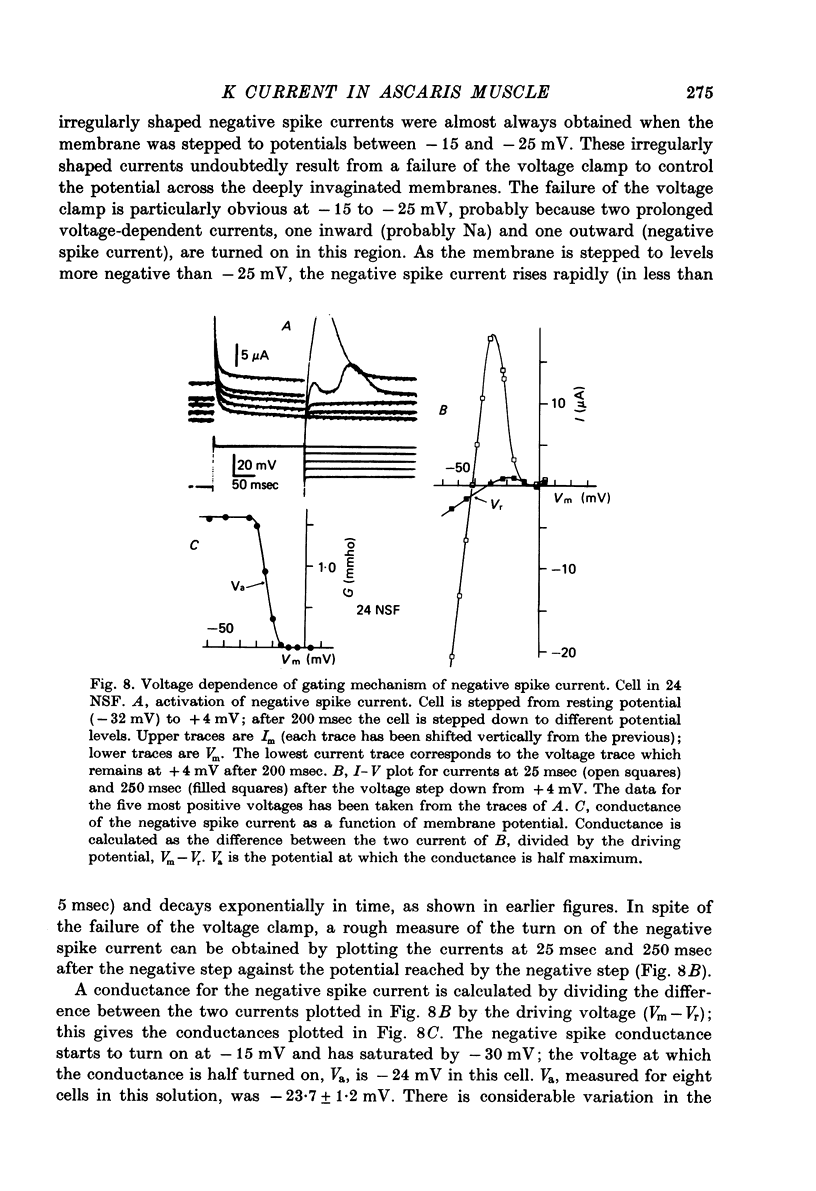
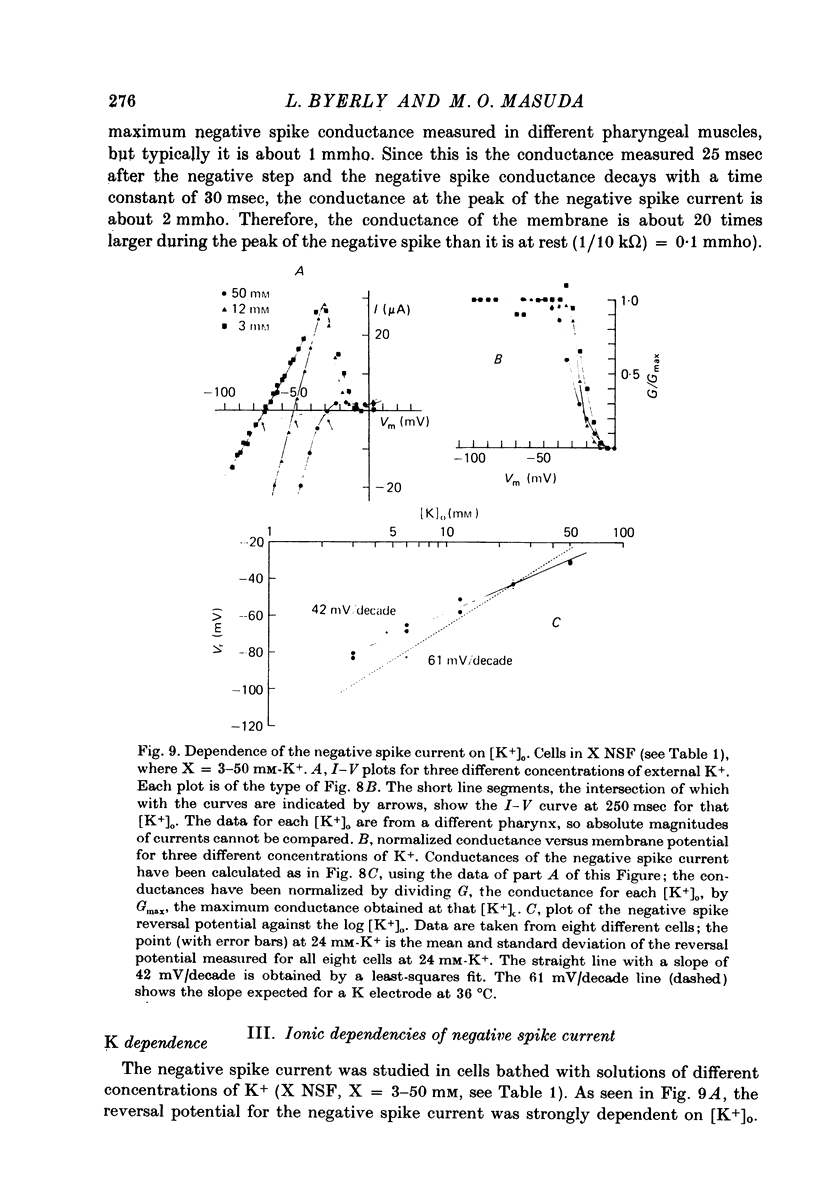
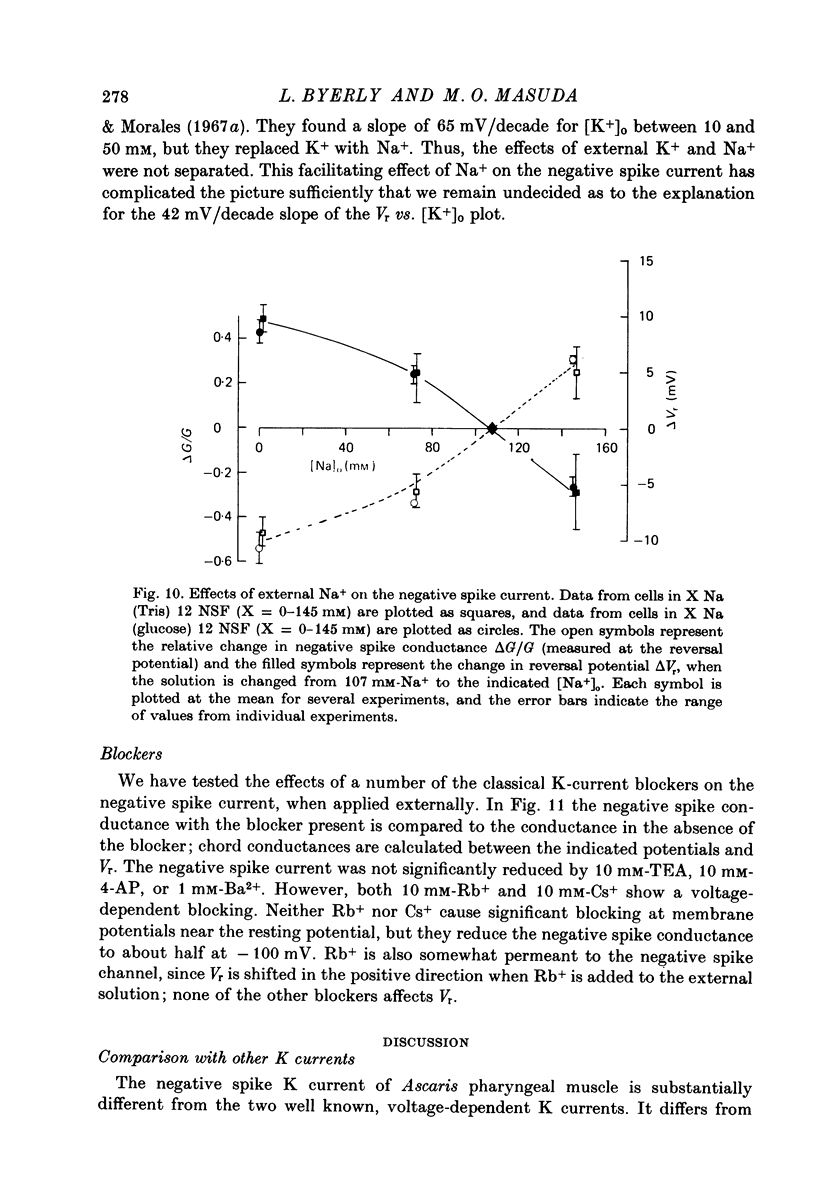
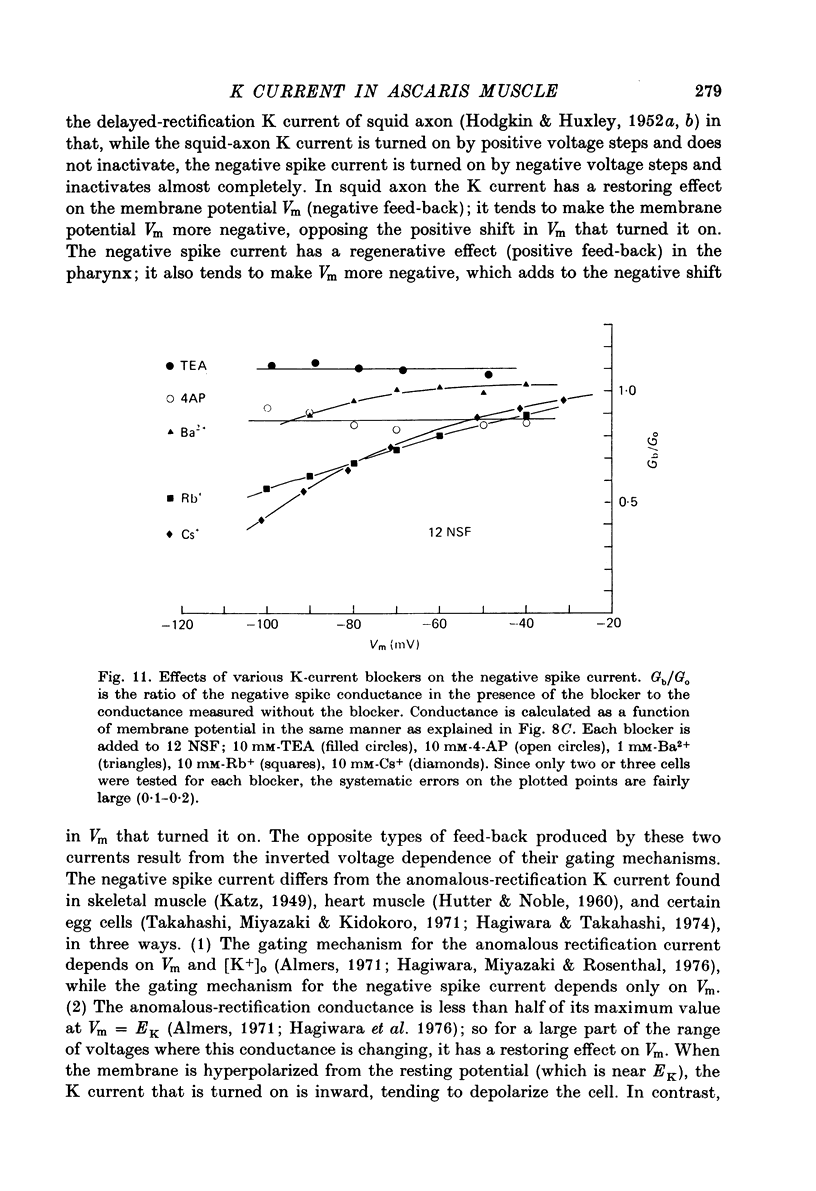
1. A voltage clamp has been developed for the pharyngeal muscle of the nematode Ascaris lumbricoides and has been used to analyse the potassium current that produces a negative-going, regenerative action potential in this muscle. 2. Depolarizing voltage steps elicit a sustained inward current; returning the membrane voltage to the resting level evokes a strong, transient, outward current. This outward current reverses direction at the same voltage as that reached by the negative-going spike and is identified as the negative spike current. 3. The negative spike current decays with a time constant of 30 msec at voltages more negative than -30mV. This inactivation of the negative spike conductance is removed by holding the membrane at potentials more positive than -15mV. The time constant for removal of inactivation decreases from more than 300 msec at -15 mV to about 30 msec at +10 mV. 4. When inactivation has been removed, the negative spike conductance is turned on by stepping to potentials more negative than -15 mV. 5. Although the reversal potential for this current depends strongly on [K+]o (42 mV/decade), the potential at which the conductance is turned on is independent of [K+]o. 6. External Na+ seems to facilitate the negative spike current. Reduction of [Na+]o reduces its conductance and shifts the reversal potential to more positive values. 7. External Rb+ and Cs+ show voltage-dependent blocking of this current. 8. This K current is different from all the K currents which have been studied previously; however, it is analogous to the classical Na current of nerve and muscle, except for an inversion of the voltage dependencies.
Full text
PDF


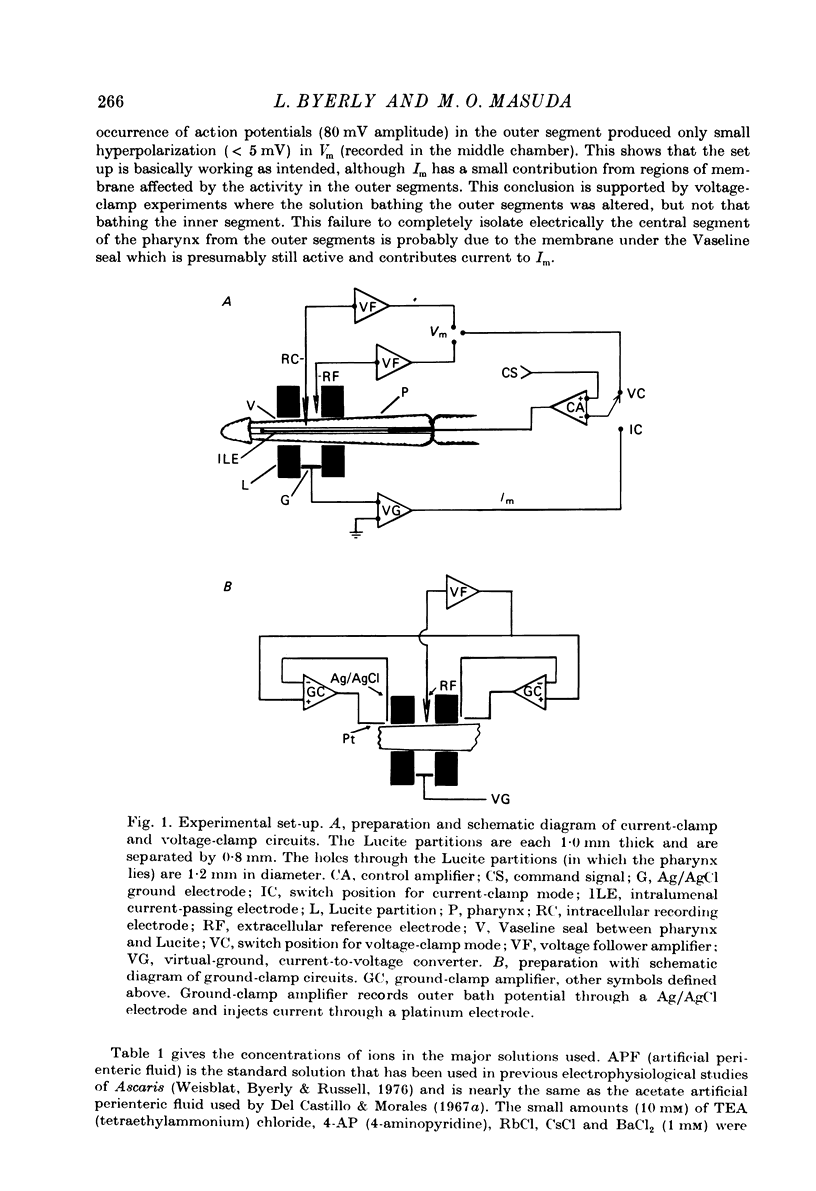

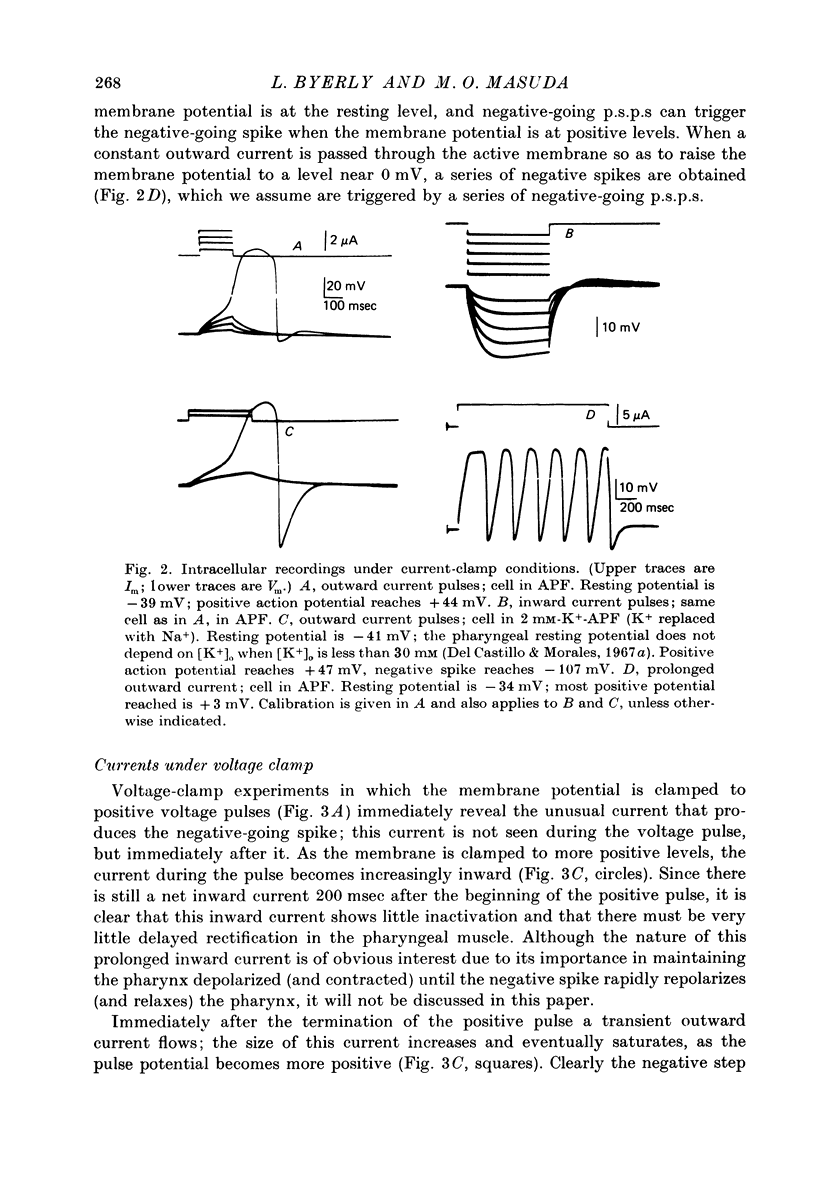
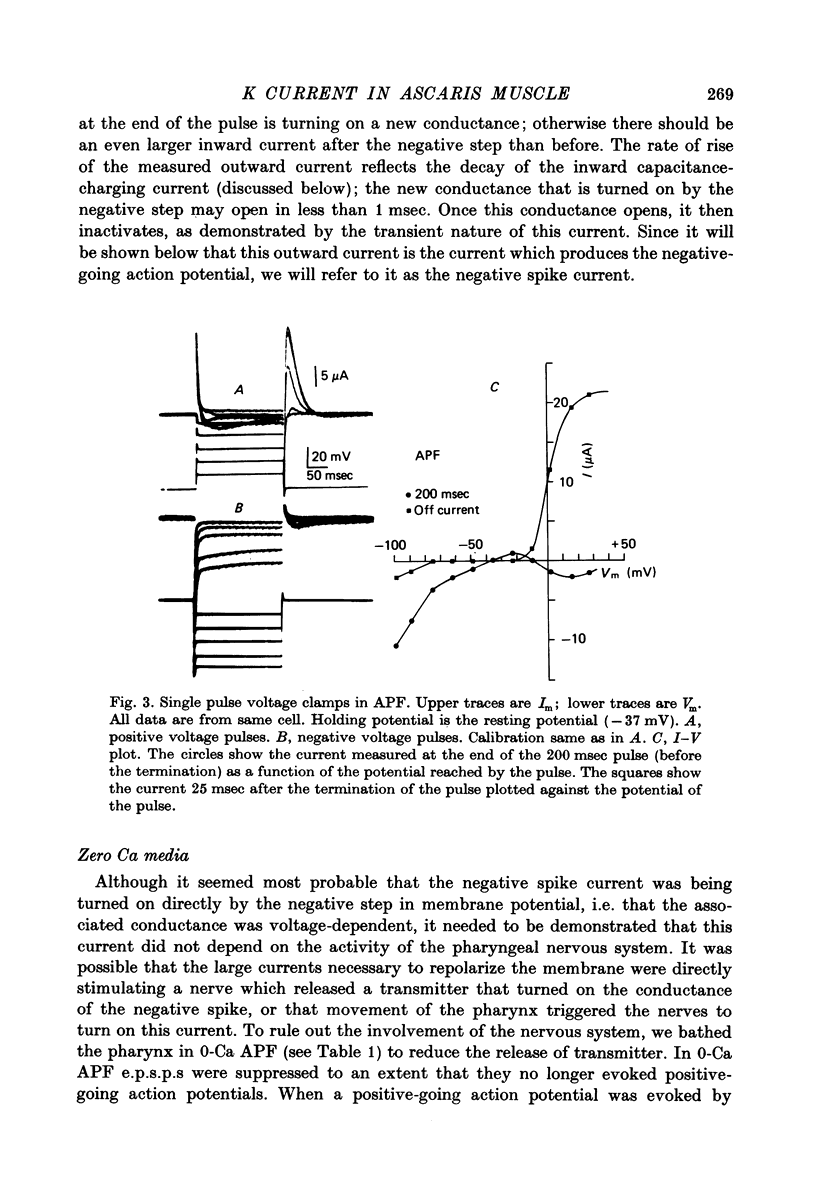
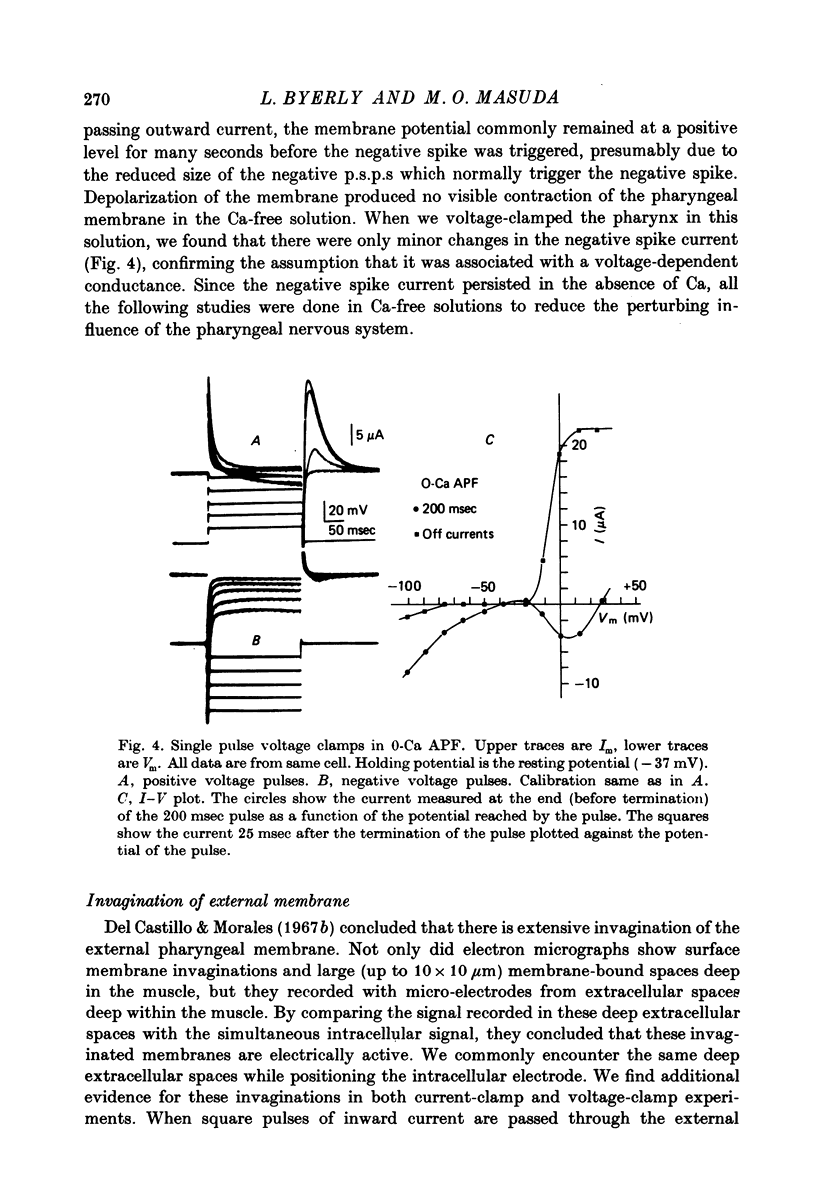






Images in this article
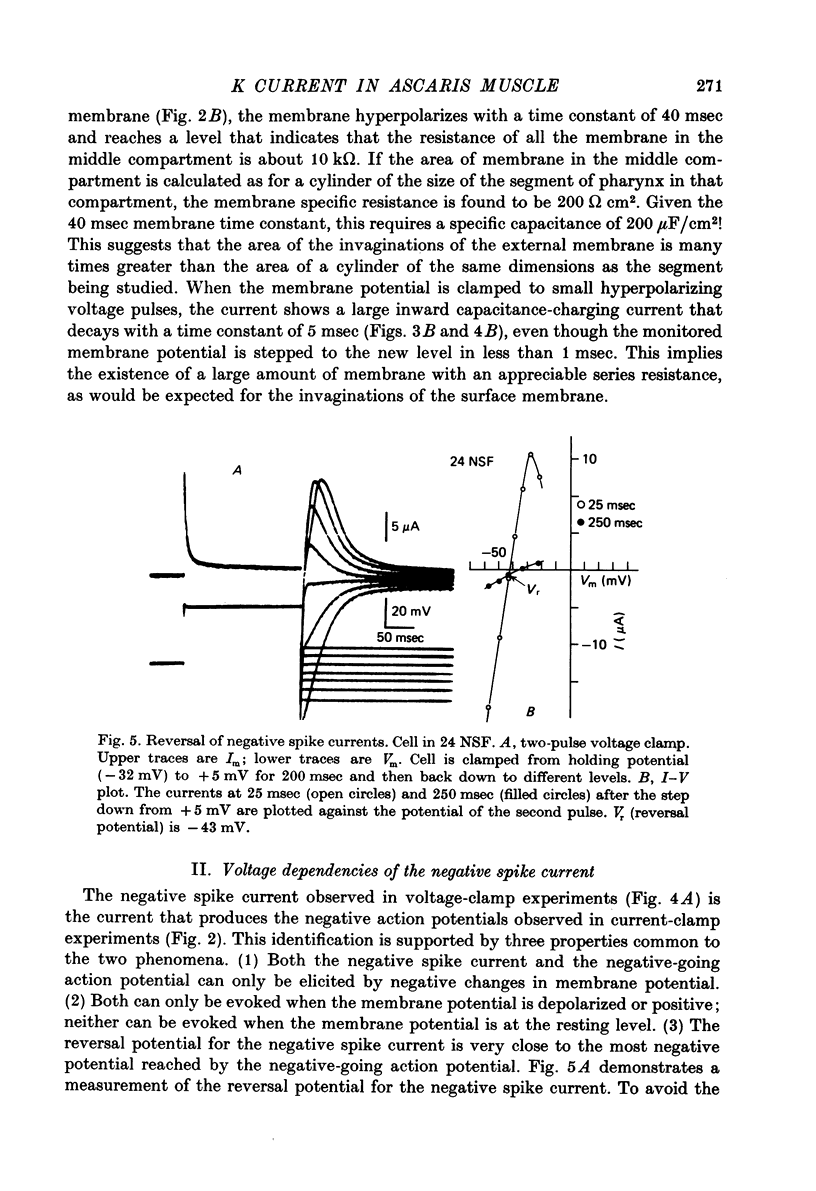
Selected References
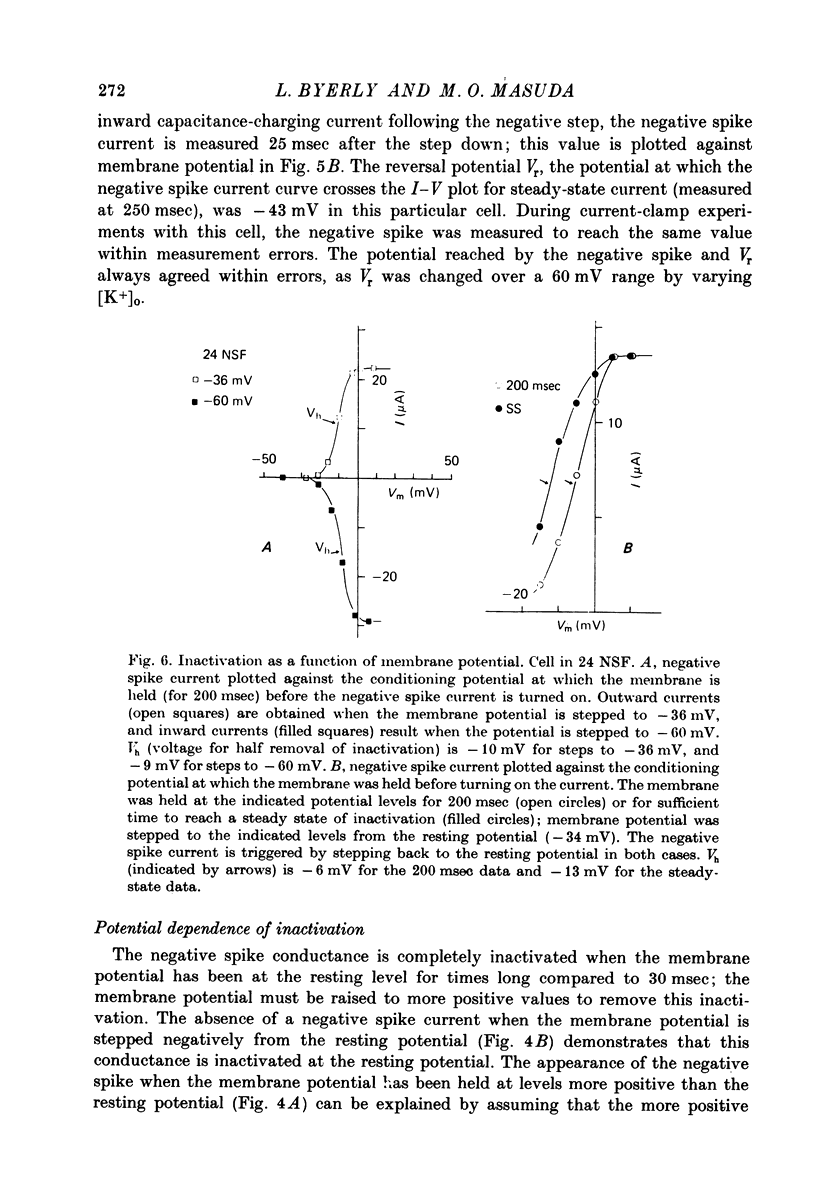
These references are in PubMed. This may not be the complete list of references from this article.
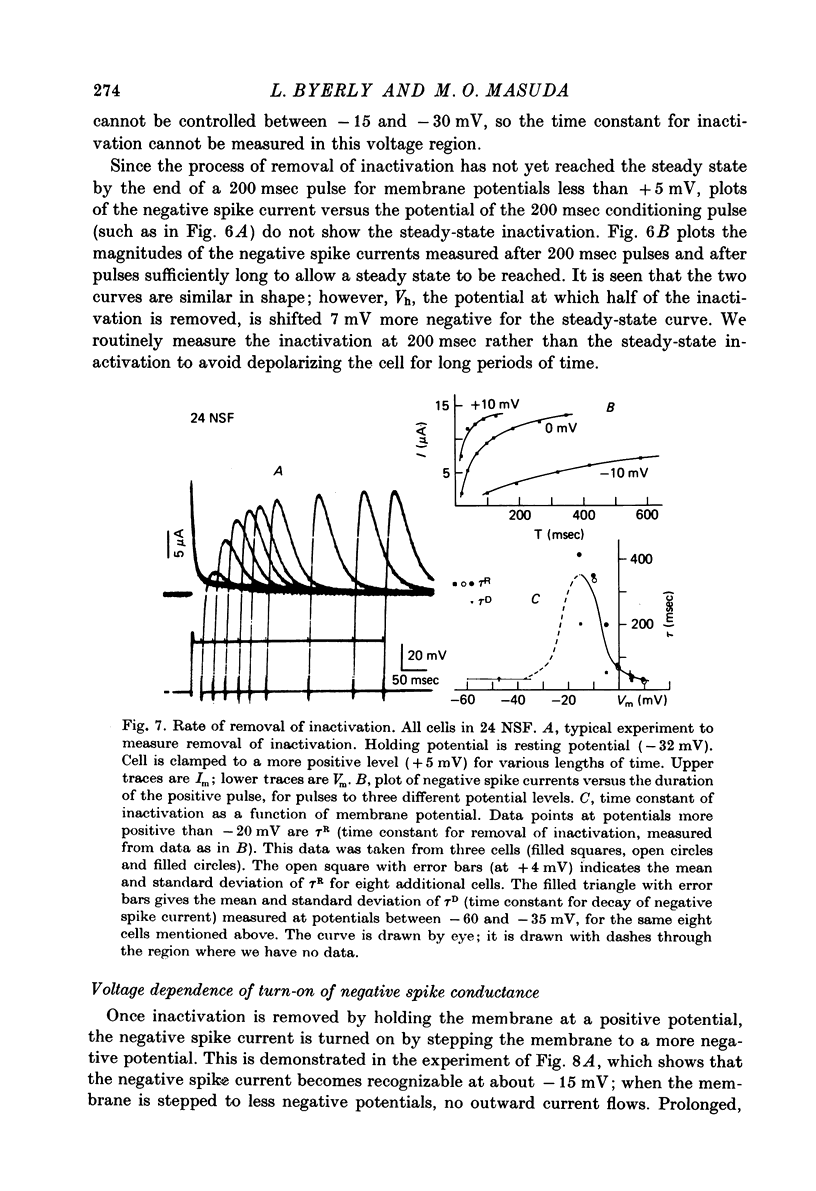
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. The decline of potassium permeability during extreme hyperpolarization in frog skeletal muscle. J Physiol. 1972 Aug;225(1):57–83. doi: 10.1113/jphysiol.1972.sp009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W., MORALES T. HYPERPOLARIZING ACTION POTENTIALS RECORDED FROM THE OESOPHAGUS OF ASCARIS LUMBRICOIDES. Nature. 1964 Aug 1;203:530–531. doi: 10.1038/203530a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- MAPES C. J. STRUCTURE AND FUNCTION IN THE NEMATODE PHARYNX. I. THE STRUCTURE OF THE PHARYNGES OF ASCARIS LUMBRICOIDES, OXYURIS EQUI, APLECTANA BREVICAUDATA AND PANAGRELLUS SILUSIAE. Parasitology. 1965 May;55:269–284. doi: 10.1017/s003118200006875x. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger J. F. The fine structure of fibrillar components and plasma membrane contacts in esophageal myoepithelium of Ascaris lumbricoides (var. suum). J Ultrastruct Res. 1966 Mar;14(5):602–617. doi: 10.1016/s0022-5320(66)80085-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Miyazaki S. I., Kidokoro Y. Development of excitability in embryonic muscle cell membranes in certain tunicates. Science. 1971 Jan 29;171(3969):415–418. doi: 10.1126/science.171.3969.415. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo J., Morales T. Extracellular action potentials recorded from the interior of the giant esophageal cell of Ascaris. J Gen Physiol. 1967 Jan;50(3):631–645. doi: 10.1085/jgp.50.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo J., Morales T. The electrical and mechanical activity of the esophageal cell of Ascaris lumbricoides. J Gen Physiol. 1967 Jan;50(3):603–629. doi: 10.1085/jgp.50.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]