Abstract
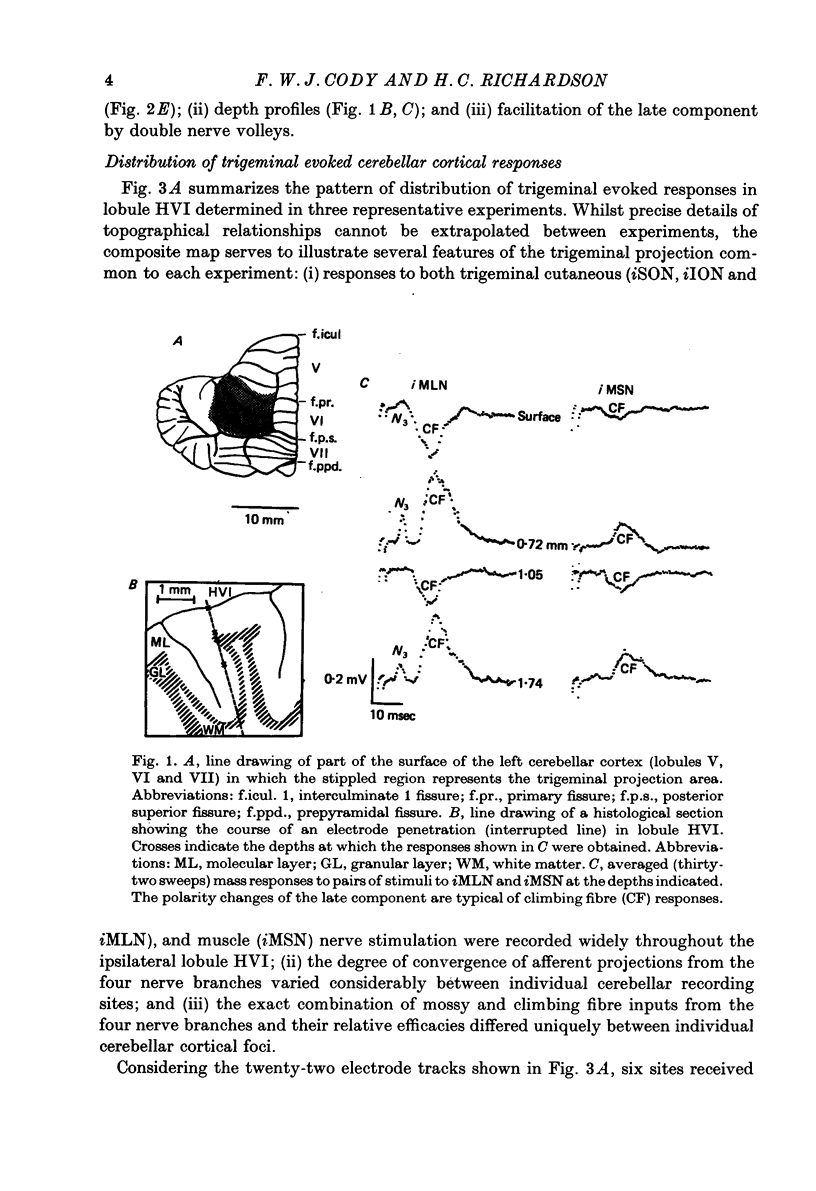
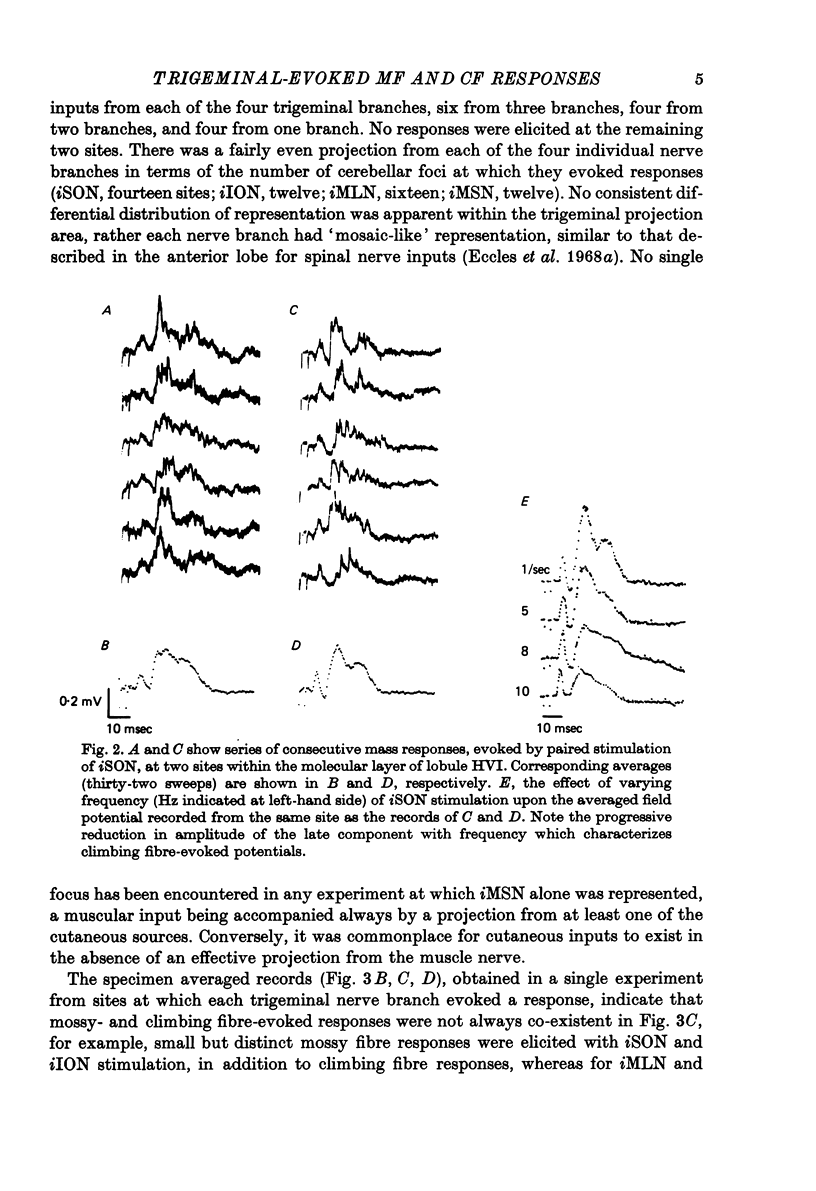
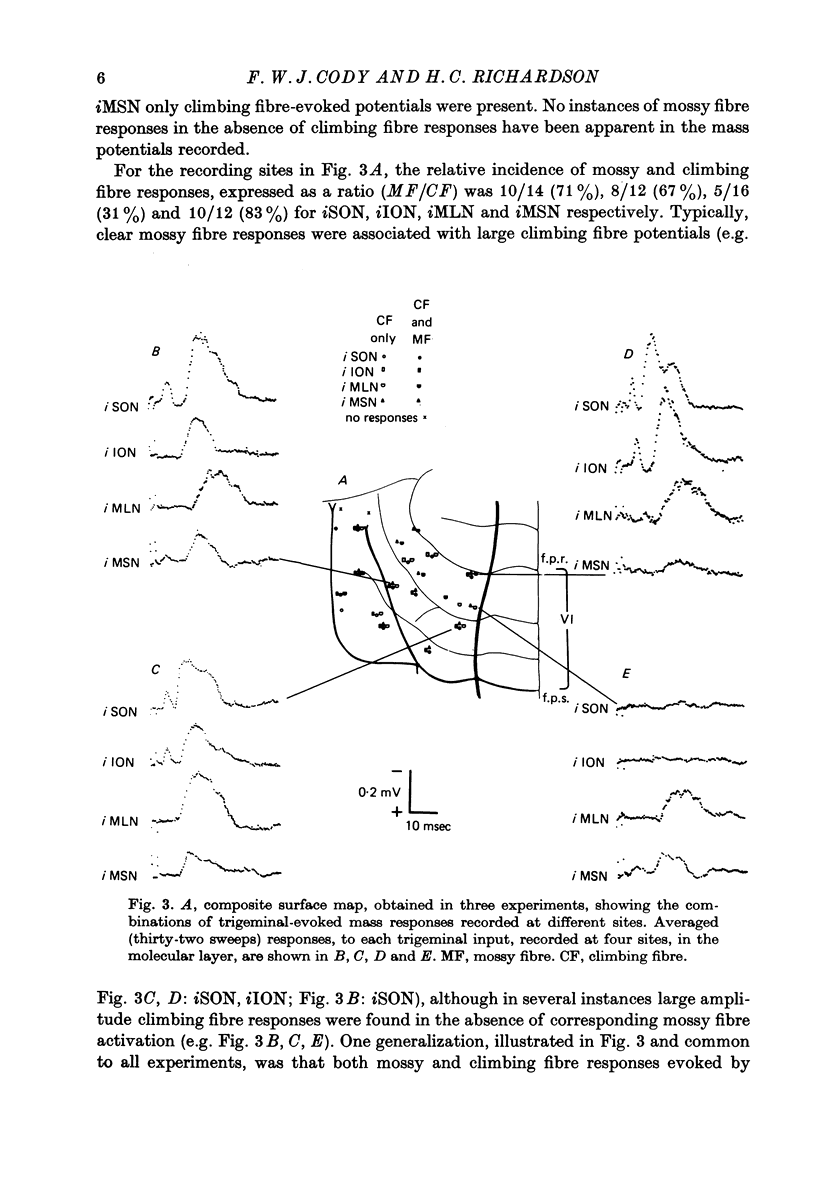
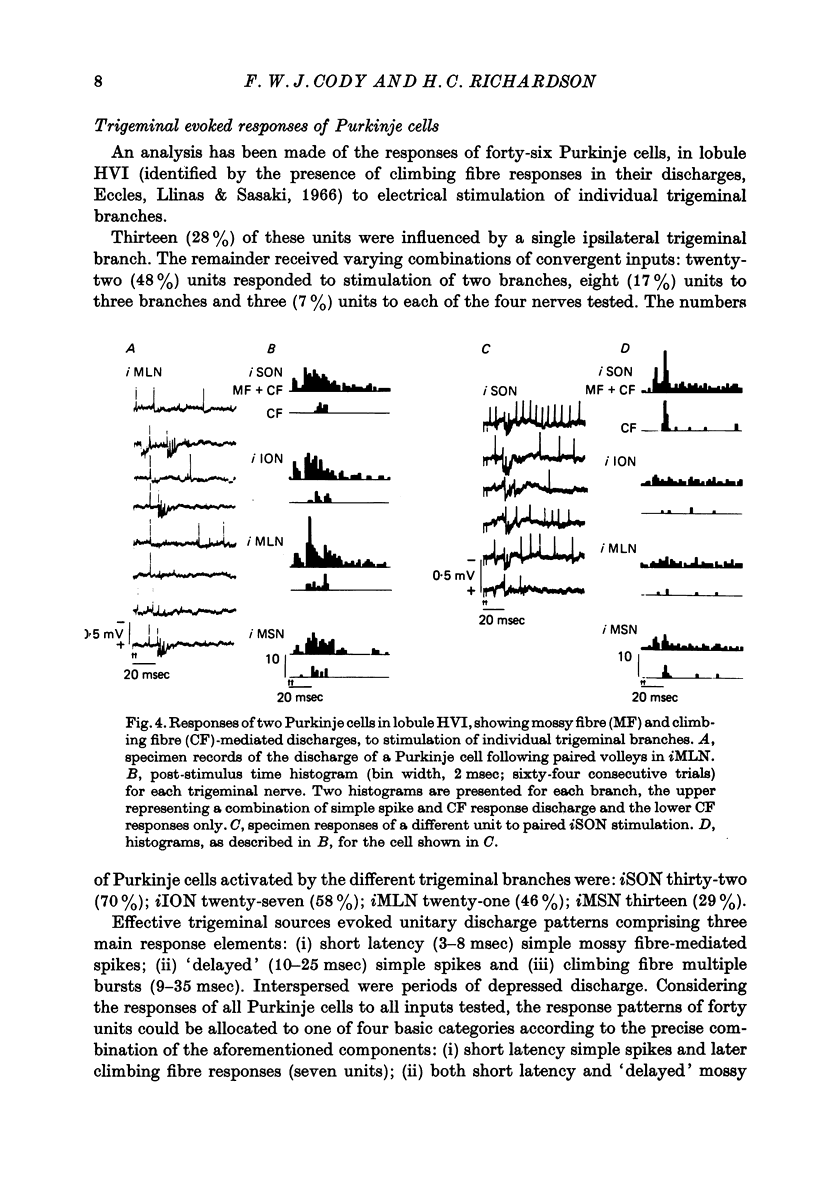
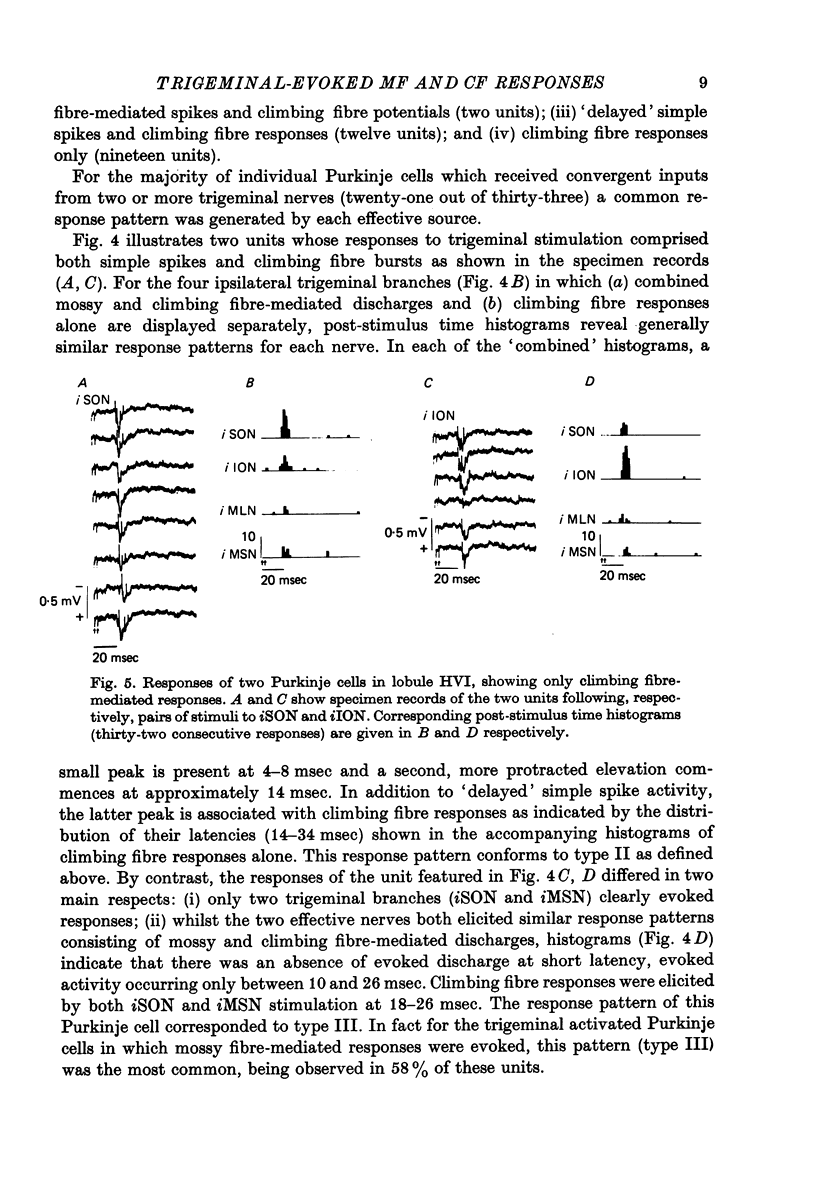
1. The trigeminal input to the cerebellar cortex was studied by recording mass and unitary resonses evoked by electrical stimulation of individual trigeminal cutaneous and muscle nerve branches, in cats lightly anaesthetized with sodium thiopentone. 2. The trigeminal projection area of the cerebellar cortex comprised essentially lobule HVI, but included adjacent folia of lobules HV and HVIIA. 3. Each trigeminal branch had a 'patchy' representation throughout the projection area and there was extensive convergence of individual afferents at each site. Exact combinations of convergent inputs varied between loci, the projection from the muscle nerve being weaker. No other differential representation of individual trigeminal branches was evident. 4. Responses were evoked by stimulation of both ipsi- and contralateral trigeminal afferents but contralateral projections were present at fewer sites. 5. Mass responses to stimulation of individual trigeminal branches comprised mossy fibre and climbing fibre-mediated potentials, although both components were not always present. Latencies for mossy and climbing fibre responses, evoked by ipsilateral nerve stimulation, were in the ranges 5--8 msec and 11--29 msec respectively. 6. Unitary responses of Purkinje cells activiated by trigeminal inputs also revealed convergence from individual ipsilateral afferent sources (28% influenced by one ipsilateral trigeminal branch, 48% by two branches, 17% by three branches and 7% by four branches). 7. Response patterns comprised one or more of the following: short latency (3--8 msec) simple, mossy fibre-mediated spikes, 'delayed' (10--25 msec) simple spikes and climbing fibre-mediated multiple spike bursts (9--35 msec).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Azzena G. B., Desole C., Palmieri G. Cerebellar projections of the masticatory and extraocular muscle proprioception. Exp Neurol. 1970 Apr;27(1):151–161. doi: 10.1016/0014-4886(70)90209-8. [DOI] [PubMed] [Google Scholar]
- BRODAL A., SAUGSTAD L. F. RETROGRADE CELLULAR CHANGES IN THE MESENCEPHALIC TRIGEMINAL NUCLEUS IN THE CAT FOLLOWING CEREBELLAR LESIONS. Acta Morphol Neerl Scand. 1965;6:147–159. [PubMed] [Google Scholar]
- Baker R., Precht W., Llinas R. Mossy and climbing fiber projections of extraocular muscle afferents to the cerebellum. Brain Res. 1972 Mar 24;38(2):440–445. doi: 10.1016/0006-8993(72)90728-7. [DOI] [PubMed] [Google Scholar]
- CARPENTER M. B., HANNA G. R. Fiber projections from the spinal trigeminal nucleus in the cat. J Comp Neurol. 1961 Aug;117:117–131. doi: 10.1002/cne.901170110. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Lee R. W., Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972 Oct;226(1):249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Richardson H. C. Trigeminal projections to the cerebellar cortex in the cat [proceedings]. J Physiol. 1977 May;267(1):41P–42P. [PubMed] [Google Scholar]
- Coffey G. L., Godwin-Austen R. B., Macgillivray B. B., Sears T. A. The form and distribution of the surface evoked responses in cerebellar cortex from intercostal nerves in the cat. J Physiol. 1971 Jan;212(1):129–145. doi: 10.1113/jphysiol.1971.sp009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. R., Wiesendanger M. Input from trigeminal cutaneous afferents to neurones of the inferior olive in rats. Exp Brain Res. 1976 Sep 24;26(2):193–202. doi: 10.1007/BF00238283. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I., MAYDAY G. Somatotopic organization within the brain-stem trigeminal complex of the cat. Exp Neurol. 1960 Jun;2:290–309. doi: 10.1016/0014-4886(60)90015-7. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G. SECONDARY NEURONES WITHIN A TRIGEMINO-CEREBELLAR PROJECTION TO THE ANTERIOR LOBE OF THE CEREBELLUM IN THE CAT. J Physiol. 1964 Jan;170:53–68. doi: 10.1113/jphysiol.1964.sp007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PROCTOR R., RYAN R. D. A SINGLE-NEURONE INVESTIGATION OF SOMATOTOPIC ORGANIZATION WITHIN THE CAT'S TRIGEMINAL BRAIN-STEM NUCLEI. J Physiol. 1963 Aug;168:147–157. doi: 10.1113/jphysiol.1963.sp007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkynè cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Topographical investigations on the climbing fiber inputs from forelimb and hindlimb afferents to the cerebellar anterior lobe. Exp Brain Res. 1968;6(3):195–215. doi: 10.1007/BF00235124. [DOI] [PubMed] [Google Scholar]
- Gordon M., Rubia F. J., Strata P. The effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973 Mar 29;17(1):50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Keller O., Vyklicky L., Sykova E. Reflexes from A delta and A alpha trigeminal afferents. Brain Res. 1972 Feb 25;37(2):330–332. doi: 10.1016/0006-8993(72)90680-4. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Latham A., Paul D. H. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971 Feb;213(1):135–156. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Miles T. S., Wiesendanger M. Climbing fibre inputs to cerebellar Purkinje cells from trigeminal cutaneous afferents and the SI face area of the cerebral cortex in the cat. J Physiol. 1975 Feb;245(2):425–445. doi: 10.1113/jphysiol.1975.sp010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles T. S., Wiesendanger M. Organization of climbing fibre projections to the cerebellar cortex from trigeminal cutaneous afferents and from the SI face area of the cerebral cortex in the cat. J Physiol. 1975 Feb;245(2):409–424. doi: 10.1113/jphysiol.1975.sp010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the ventral spino-olivocerebellar path. J Physiol. 1968 May;196(2):453–478. doi: 10.1113/jphysiol.1968.sp008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART W. A., KING R. B. FIBER PROJECTIONS FROM THE NUCLEUS CAUDALIS OF THE SPINAL TRIGEMINAL NUCLEUS. J Comp Neurol. 1963 Oct;121:271–286. doi: 10.1002/cne.901210209. [DOI] [PubMed] [Google Scholar]
- Sessle B. J. Modulation of alpha and gamma trigeminal motoneurons by various peripheral stimuli. Exp Neurol. 1977 Feb;54(2):323–339. doi: 10.1016/0014-4886(77)90273-4. [DOI] [PubMed] [Google Scholar]
- Thexton A. J. Jaw opening and jaw closing reflexes in the cat. J Physiol. 1968 Jul;197(1):34P–35P. [PubMed] [Google Scholar]


