Abstract
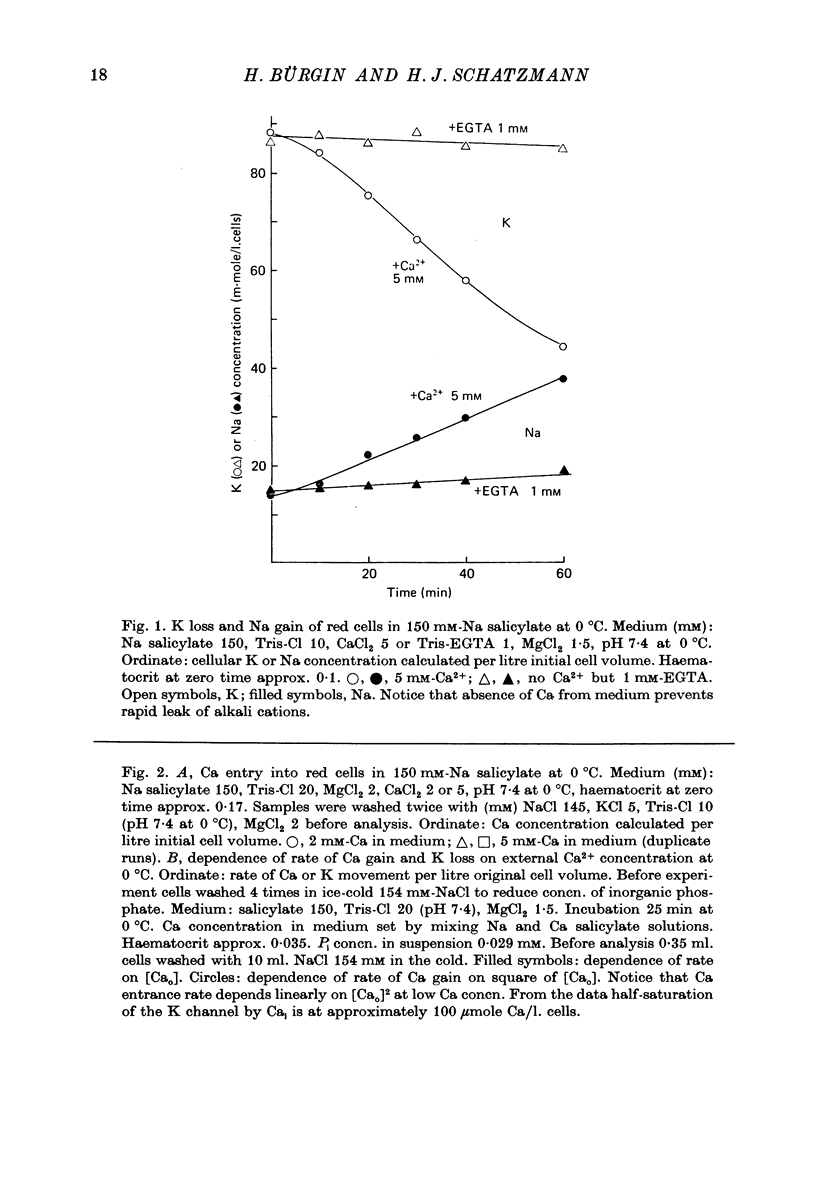
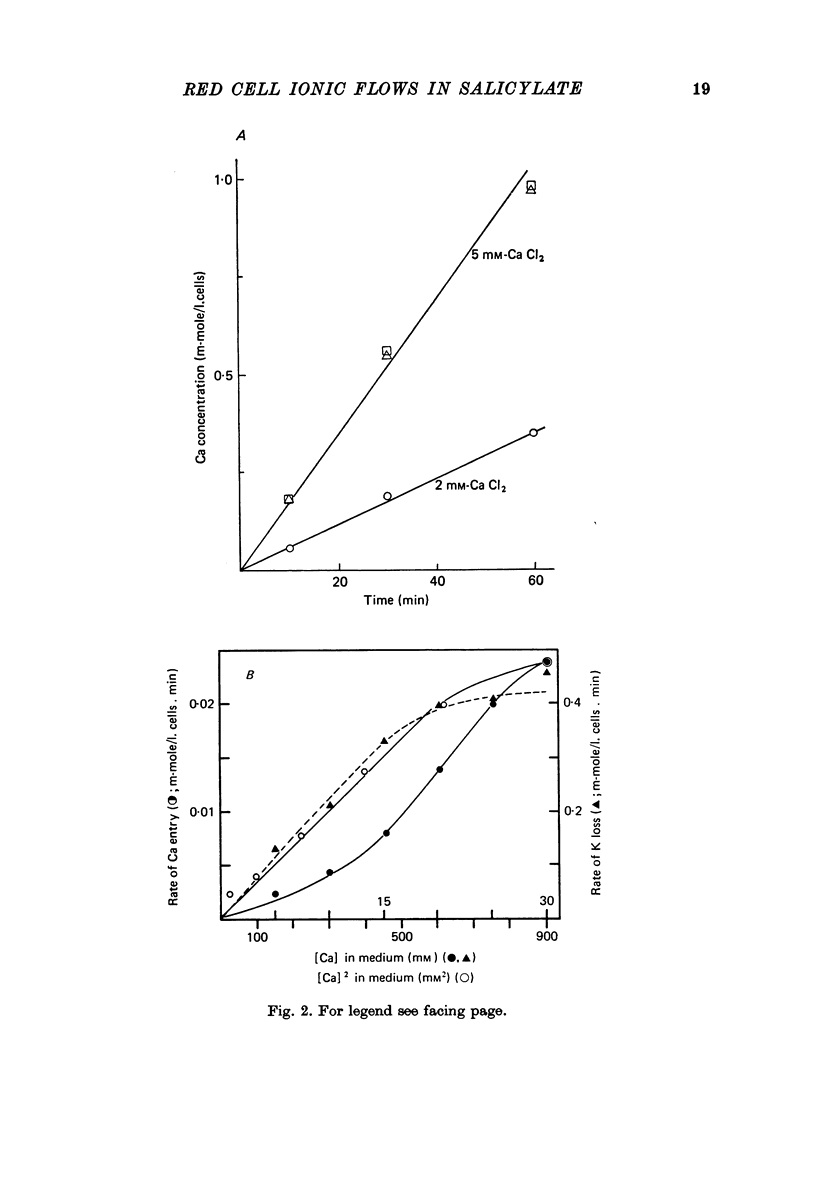
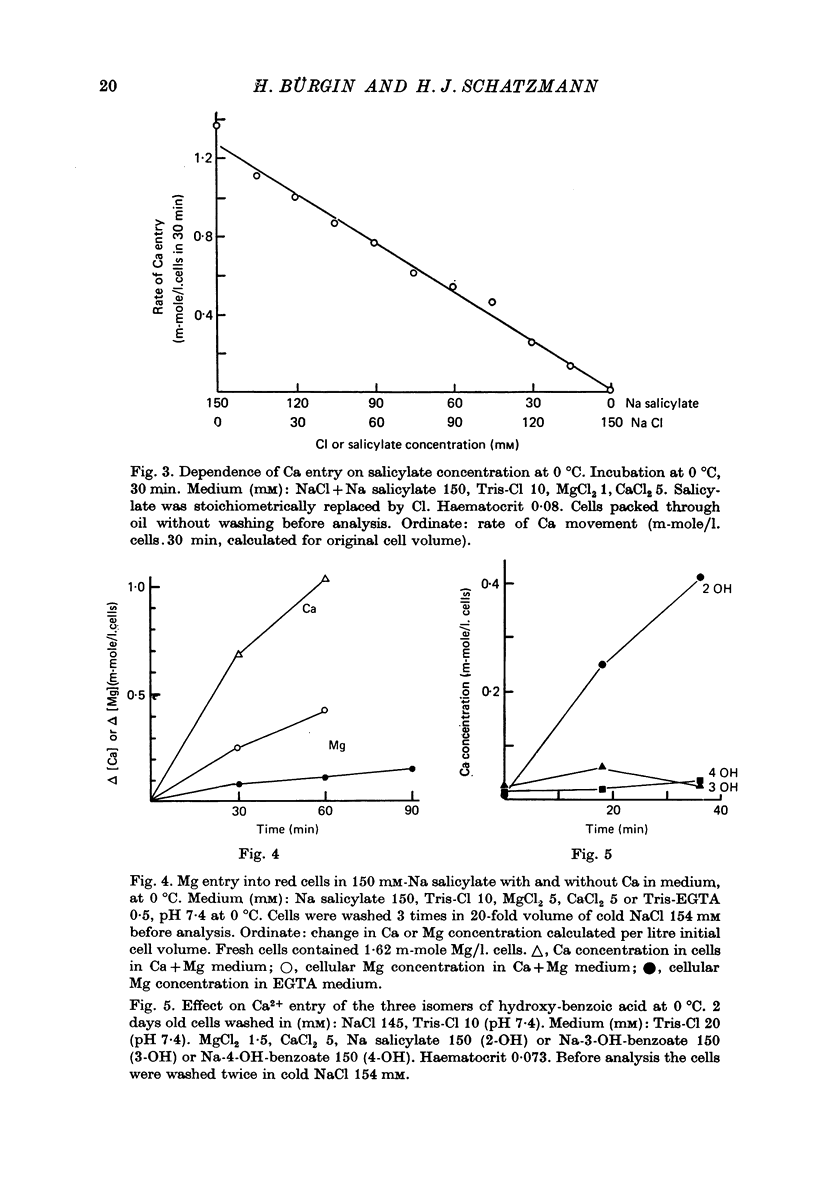
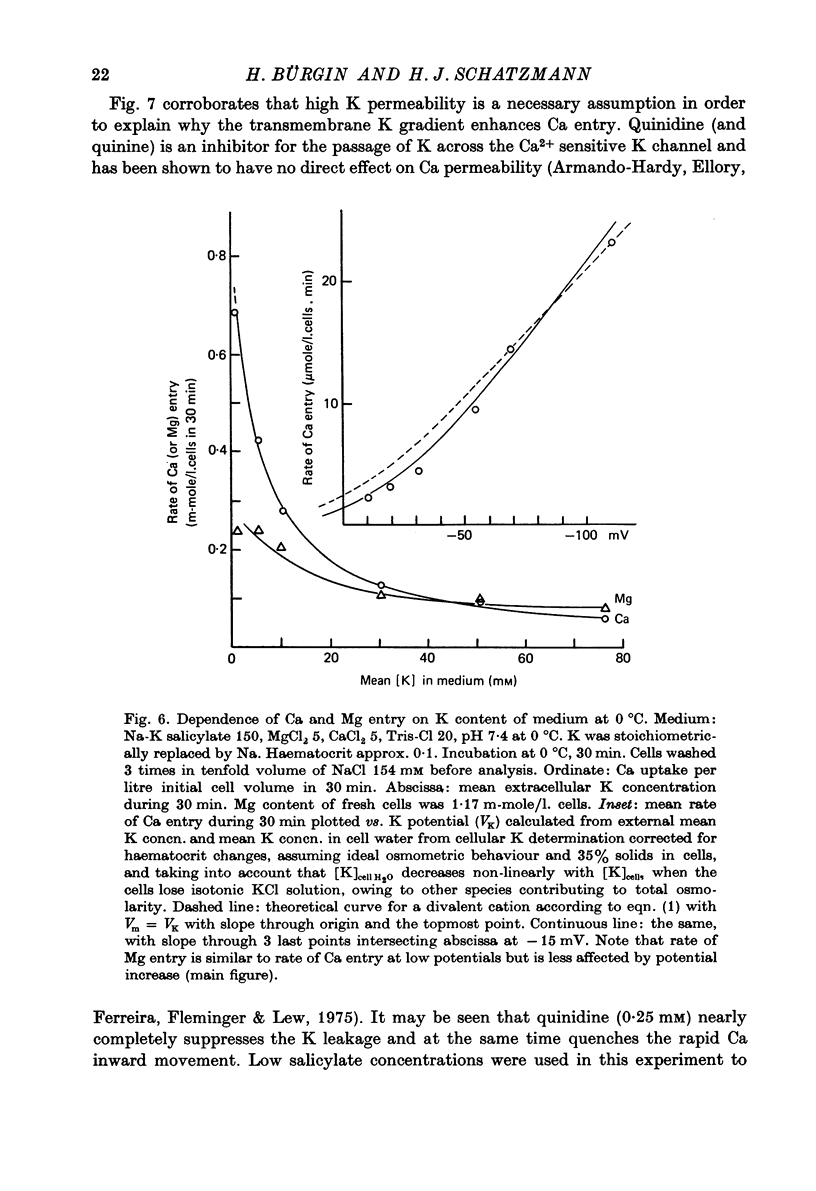
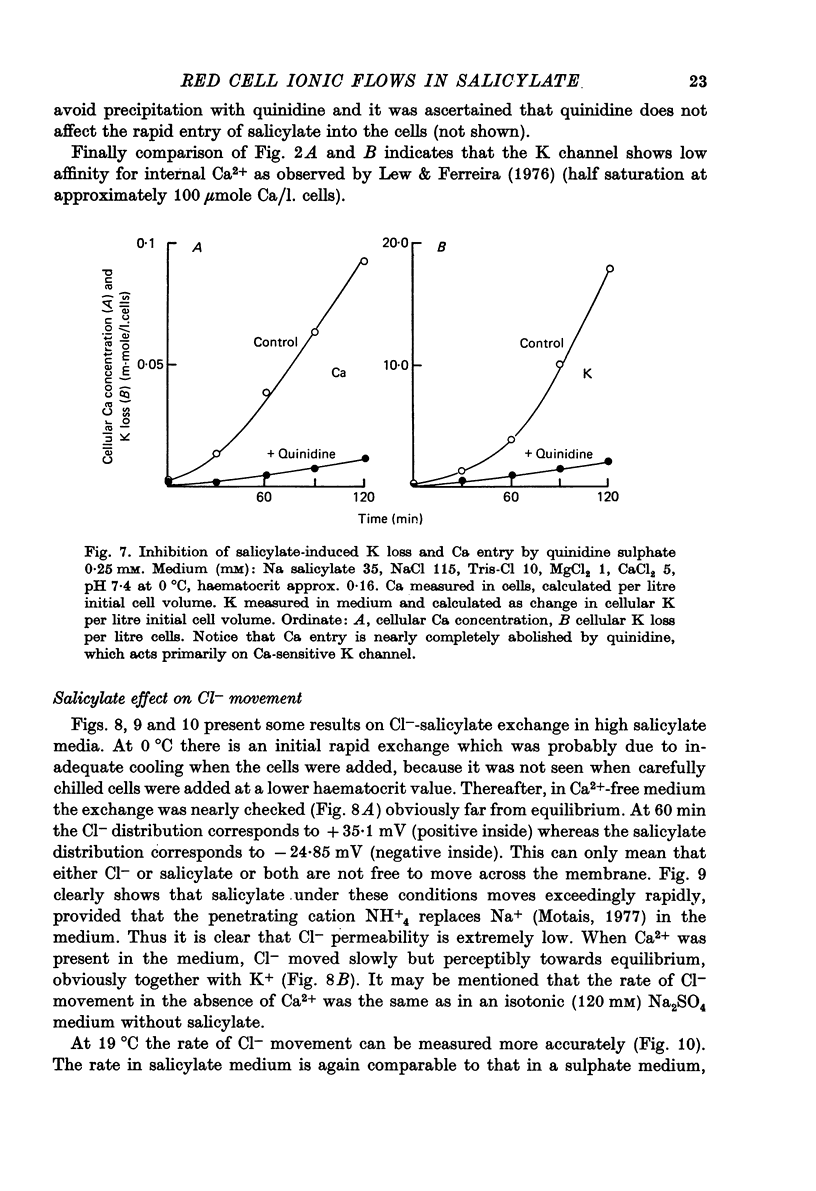
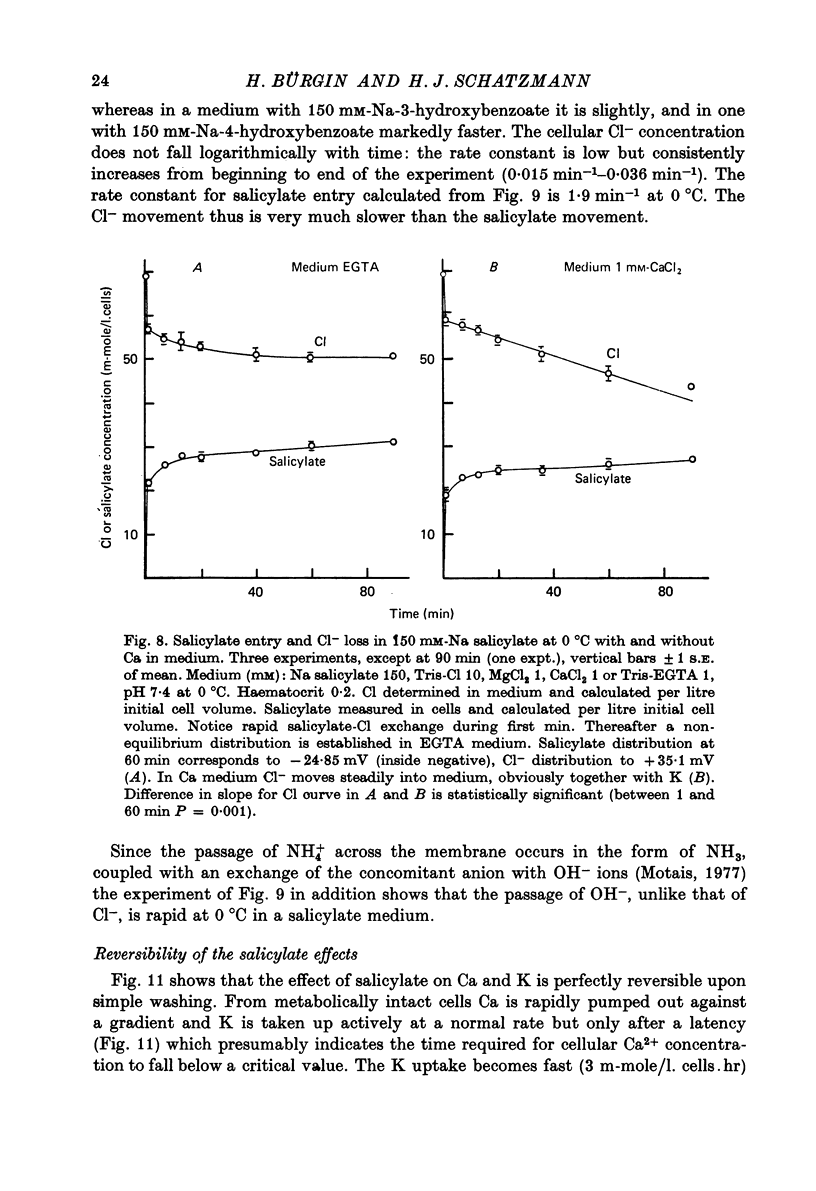
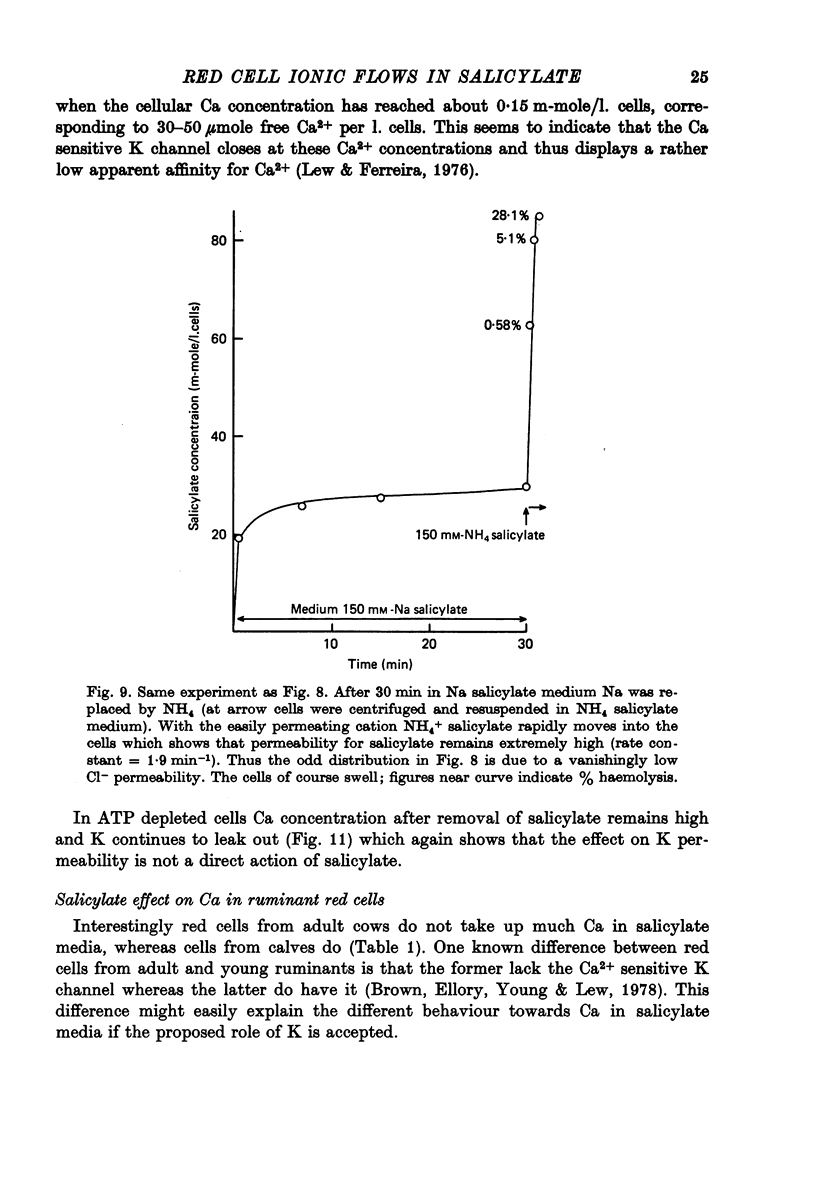
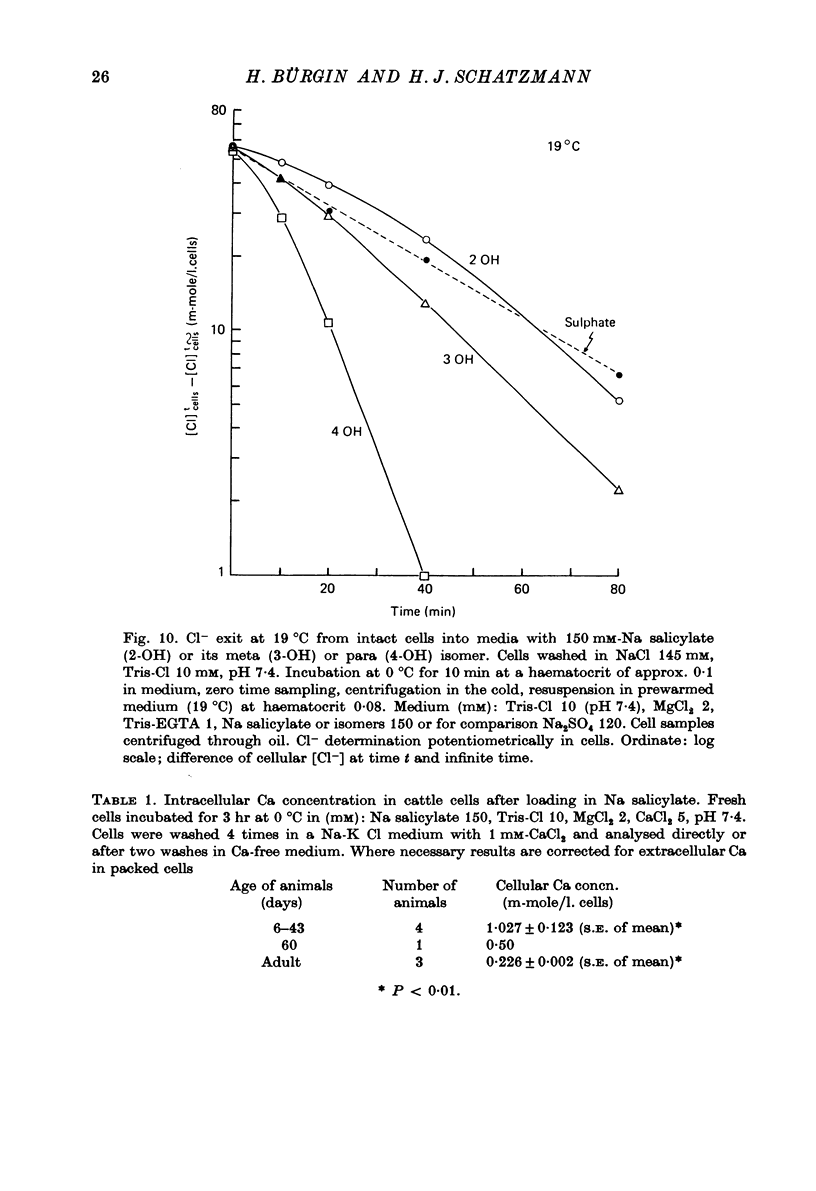
1. From a 150 mM-NH4 salicylate medium salicylate enters human red cells with a rate constant of 1.9 min-1 at 0 degrees C. 2. Salicylate increases the red cell permeability for Ca2+ (and Mg2+). There is no saturation of the Ca2+ transfer with respect to salicylate up to 150 mM and with respect to external Ca2+ up to 30 mM. 3. Ca2+ entering from salicylate media activates the Ca-sensitive K channel present in human but not in adult ruminant red cells. 4. The increase in K permeability which ensues hyperpolarizes the membrane in Na salicylate media, accelerating further Ca2+ entry and Mg2+ entry and favouring Cl- loss (see Fig. 8). The Ca2+ inward movement is in agreement with the constant field equation if the membrane potential is assumed to equal the K equilibrium potential and if two charges are attributed to the mobile species. 5. The effect of salicylate on Ca2+ permeability and hence its sequelae are reversible upon washing the cells. 6. 3-OH-benzoic acid and 4-OH-benzoic acid do not exert the effect salicylate has on Ca2+ permeability. 7. In 150 mM-Na salicylate media the Cl--salicylate exchange is virtually nil at 0 degrees C. The exchange seen at 19 degrees C is obviously not across the anion exchange mechanism and proceeds at a rate comparable to that for Cl- movement in the nonexchange-restricted mode given by Hunter (1971, 1977) for cells in a normal medium. 8. Ca2+ seems to increase the Cl- permeability seen under these conditions. 9. The possibility that salicylate acts as an ionophore for Ca2+ is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armando-Hardy M., Ellory J. C., Ferreira H. G., Fleminger S., Lew V. L. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol. 1975 Aug;250(1):32P–33P. [PubMed] [Google Scholar]
- Blum R. M., Hoffman J. F. Ca-induced K transport in human red cells: localization of the Ca-sensitive site to the inside of the membrane. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1146–1152. doi: 10.1016/s0006-291x(72)80094-9. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Ellory J. C., Young J. D., Lew V. L. A calcium-activated potassium channel present in foetal red cells of the sheep but absent from reticulocytes and mature red cells. Biochim Biophys Acta. 1978 Aug 4;511(2):163–175. doi: 10.1016/0005-2736(78)90311-5. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Use of ionophore A23187 to measure and to control free and bound cytoplasmic Mg in intact red cells. Nature. 1977 May 26;267(5609):360–362. doi: 10.1038/267360a0. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. J. A quantitative estimate of the non-exchange-restricted chloride permeability of the human red cell. J Physiol. 1971 Oct;218 (Suppl):49P–50P. [PubMed] [Google Scholar]
- Hunter M. J. Human erythrocyte anion permeabilities measured under conditions of net charge transfer. J Physiol. 1977 Jun;268(1):35–49. doi: 10.1113/jphysiol.1977.sp011845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Fuhrmann G. F., Rothstein S., Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977 Mar;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Riordan J. R., Schuhmann B., Wood-Guth I., Passow H. Calcium-potassium-stimulated net potassium efflux from human erythrocyte ghosts. J Membr Biol. 1975 Dec 4;25(1-2):1–22. doi: 10.1007/BF01868565. [DOI] [PubMed] [Google Scholar]
- Lassen U. V., Pape L., Vestergaard-Bogind B. Chloride conductance of the amphiuma red cell membrane. J Membr Biol. 1978 Feb 6;39(1):27–48. doi: 10.1007/BF01872753. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Ferreira H. G. Variable Ca sensitivity of a K-selective channel in intact red-cell membranes. Nature. 1976 Sep 23;263(5575):336–338. doi: 10.1038/263336a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. Salicylates and phospholipid bilayer membranes. Nature. 1973 May 25;243(5404):234–236. doi: 10.1038/243234a0. [DOI] [PubMed] [Google Scholar]
- Passow H., Schnell K. F. Chemical modifiers of passive ion permeability of the erythrocyte membrane. Experientia. 1969 May 15;25(5):460–468. doi: 10.1007/BF01900757. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gerlóczy A., Gárdos G. Transport parameters and stoichiometry of active calcium ion extrusion in intact human red cells. Biochim Biophys Acta. 1977 Jan 4;464(1):93–107. doi: 10.1016/0005-2736(77)90373-x. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. The use of ionophores of rapid loading of human red cells with radioactive cations for cation-pump studies. J Membr Biol. 1976 May;26(4):357–370. doi: 10.1007/BF01868883. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Calcium-dependent potassium exchange in human red cell ghosts. J Physiol. 1976 Mar;256(1):227–244. doi: 10.1113/jphysiol.1976.sp011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. The preparation of human red cell ghosts containing calcium buffers. J Physiol. 1976 Mar;256(1):209–225. doi: 10.1113/jphysiol.1976.sp011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Paradoxical temperature dependence of sodium and potassium fluxes in human red cells. J Physiol. 1970 May;207(3):563–580. doi: 10.1113/jphysiol.1970.sp009081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Gill F. M. Red cell calcium leak in congenital hemolytic anemia with extreme microcytosis. Blood. 1976 Feb;47(2):197–210. [PubMed] [Google Scholar]


