Abstract
Existing evidence suggests that oxidative insults and antioxidant defense mechanisms play a critical role in the host cell response during infection of endothelial cells by Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever. Heme oxygenase (HO), a rate-limiting enzyme in the pathway for heme catabolism, protects against oxidant damage in a variety of stress situations. Here, we report on the expression of the inducible and constitutive HO isozymes, HO-1 and HO-2, during R. rickettsii infection of endothelial cells. Steady-state levels for HO-1 mRNA were increased two- to threefold, as early as 4 h postinfection, whereas HO-2 mRNA was not affected. Induction of HO-1 mRNA was dependent on the dose of infection and occurred in a time-dependent manner, reaching maximal levels at 4 to 7 h. The increase in HO-1 mRNA occurred at the level of trancription as it was blocked by the transcriptional inhibitors, actinomycin D and α-amanitin. The eukaryotic protein synthesis inhibitor, cycloheximide, caused a >50% reduction in the infection-induced increase in HO-1 mRNA level, suggesting its dependence on de novo protein synthesis of host cell. The uptake of viable organisms appeared to be necessary, since inactivation of R. rickettsii by heat or formalin fixation, or incubation of cells with cytochalasin B to prevent entry resulted in marked inhibition of HO-1 response. N-Acetyl-l-cysteine, a known oxidant scavenger, inhibited the HO-1 induction by R. rickettsii. Finally, Western analysis with a specific monoclonal antibody revealed higher levels of HO-1 protein (∼32 kDa), confirming that changes in HO-1 mRNA levels were followed by increases in the levels of protein. The findings indicate that R. rickettsii infection induces HO-1 expression in host endothelial cells and suggest an important role for this enzyme in cellular response to infection, possibly by serving a protective function against oxidative injury.
Rickettsiae are obligate, intracellular parasites and causative agents of severe bacterial diseases of humans, including epidemic typhus and Rocky Mountain spotted fever, caused, respectively, by Rickettsia prowazekii and R. rickettsii. These bacteria are transmitted to their mammalian hosts by arthropod vectors such as ticks, fleas, lice, and mites and grow within the cytoplasm (and occasionally the nucleus) of eukaryotic cells (37, 62). Rickettsiae exhibit a tropism for the endothelium and invade vascular endothelial cells as a major target. The proliferation of organisms in capillary endothelium and associated cytopathic effects of infection contribute to increased vascular permeability (57). Studies have suggested that the host endothelial cells actively respond to intracellular infection by altering the expression of several proteins, including tissue factor (50, 56), plasminogen activator inhibitor 1 (13, 44), E-selectin (12, 49), interleukin-1 (IL-1), IL-6, and IL-8 (22, 51). Ultrastructural studies have shown that infected endothelial cells sustain severe damage at late stages of infection, as indicated by dilatation of the rough endoplasmic reticulum and outer nuclear envelope, loss of osmoregulatory control, and cell lysis (45, 46).
The mechanisms of cell injury induced by R. rickettsii remain largely unknown. Based on the striking architectural changes in the cytoskeleton of infected cells, it was speculated that reactive oxygen species may be one of the major causes of cell injury by R. rickettsii. Subsequent biochemical analysis confirmed the accumulation of intracellular peroxides and superoxide radicals (42, 47), detection of higher amounts of extracellular hydrogen peroxide in the culture medium of infected cells (21), and reduction in levels of intracellular thiols (48). Further, the activities of three important enzymes of the cellular antioxidant system, namely, glucose-6-phosphate dehydrogenase, catalase, and glutathione peroxidase, were downregulated, while that of superoxide dismutase was increased in endothelial cells exposed to R. rickettsii (11, 42). α-Lipoic acid, a lipoamide compound with potent antioxidant properties, exhibited a protective effect against oxidative changes by decreasing the levels of peroxides and elevating reduced glutathione and glutathione peroxidase activity (15). These observations with human umbilical vein endothelial cells and similar studies utilizing a permanent endothelial cell line, EA.hy 926 (16), provide evidence for oxidative stress during R. rickettsii infection, which may be responsible for cell injury.
The cellular response to oxidative stress-inducing agents such as heavy metals, UV irradiation, heme, hemoglobin, and hydrogen peroxide involves the production of a number of cellular mediators, including acute-phase proteins, eicosanoids, and cytokines. Heme oxygenase (HO; EC 1.14.99.3), the initial and rate-limiting enzyme in the pathway for heme catabolism, plays a vital role in diverse biological processes, including cell respiration, energy generation, oxidative biotransformation, and cell growth and differentiation (2, 7). HO is present in most mammalian tissues and catalyzes the degradation of heme to biliverdin, releasing equimolar amounts of biliverdin IXa, iron, and carbon monoxide (CO) (28). Biliverdin is subsequently converted into bilirubin by the enzyme biliverdin reductase. The HO system consists of at least three isozymes. Two of them, HO-1 and HO-2, are products of distinct genes; differ in their tissue distribution and regulation and have been characterized in detail. Of these, HO-1 is inducible and designated a stress response protein, whereas HO-2 is predominantly constitutive (28). A third isozyme, HO-3, which is closely related to HO-2, has been described recently (29). Although heme is purported to be the typical HO-1 inducer, the inflammatory cytokines IL-1α and tumor necrosis factor-α (TNF-α) have been shown to be effective inducers of HO-1 in cultured human EC (54). The expression of HO-1 is sensitive to induction by oxidants, and recent work has demonstrated that HO-1 provides cytoprotective effects in models of oxidant-induced cellular and tissue injury (34). Increased HO-1 activity enhances the survival of EC exposed to heme iron (1), and bilirubin, a potent antioxidant, also protects against hydrogen peroxide-induced toxicity in an aortic endothelial cell line (32). A recent study has demonstrated that CO generated through the action of HO-1 acts as an antiapoptotic molecule, and induction of HO-1 is able to prevent a series of inflammatory reactions associated with apoptosis (4). Since HO activity is known to protect EC in response to oxidative stress induced by various stimuli, the present study was undertaken to investigate the regulation of endothelial HO-1 and HO-2 during R. rickettsii infection.
(A portion of this work was presented at the 101st General Meeting of the American Society for Microbiology, in Orlando, Fla., 20 to 24 May 2001.)
MATERIALS AND METHODS
Reagents.
Actinomycin D, aprotinin, cycloheximide, cytochalasin B, leupeptin, N-acetyl-l-cysteine (NAC), phenylmethylsulfonyl fluoride, and polymyxin B agarose were obtained from Sigma Chemical Co. (St. Louis, Mo.). α-Amanitin was purchased from Calbiochem (San Diego, Calif.). Stock concentrations of actinomycin D and cytochalasin B were prepared in dimethyl sulfoxide and α-amanitin, cycloheximide, and NAC were dissolved in sterile water. TRI reagent was purchased from Molecular Research Center, Inc., Cincinnati, Ohio. [α-32P]dCTP (3,000 Ci/mMol) was obtained from Dupont-NEN (Boston, Mass.).
Cell culture.
Human endothelial cell cultures were established as described previously (19, 61) by using umbilical cords collected within 48 h of delivery. Cells were cultured in McCoy's 5a medium (Flow Laboratories, McLean, Va.) containing 20% fetal bovine serum, EC growth supplement (50 μg/ml; Collaborative Research, Inc., Bedford, Mass.), and heparin (100 μg/ml; Sigma). Cells at second passage were used in all experiments and were plated so as to achieve 80 to 90% confluence after 4 to 5 days in culture. Vero C1008 (African green monkey kidney) cells (American Type Culture Collection, Rockville, Md.) were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and 1 mM glutamine.
Infection with R. rickettsii and treatment with inhibitors.
Near-confluent cell cultures were infected as described previously by using a plaque-purified seed stock (1 × 107 to 5 × 107 PFU/cm2) of the Sheila Smith strain of R. rickettsii prepared in Vero cells (52). In some experiments, R. rickettsii preparations purified by sucrose-renografin density gradient centrifugation (40) were used. EC were infected with ca. 6 × 104 to 1 × 105 PFU of R. rickettsii organisms diluted in culture medium, for each square centimeter of cell culture area. After a 2-h incubation at 37°C, the inoculum was removed, and cell monolayers were washed three times with culture medium. To study the effects of treatments, cells were incubated with the desired concentrations of the inhibitor in complete culture medium for 30 to 60 min prior to and during infection with R. rickettsii. Infection of EC, plated on Thermanox coverslips (Ted Pella, Redding, Calif.), was monitored simultaneously by indirect immunofluorescence staining with a polyclonal anti-R. rickettsii antibody (Center for Disease Control, Atlanta, Ga.) and microscopic analysis as described previously (52).
Manipulation of R. rickettsii organisms.
Heat treatment was performed by incubation of R. rickettsii preparations at 65°C for 30 min with gentle intermittent shaking. Fixation was accomplished by incubation with 3.7% (vol/vol) formaldehyde for 1 h in K36 buffer (0.1 M KCl, 0.15 M NaCl, and 0.05 M potassium phosphate buffer [pH 7.0]). The effect of these treatments on R. rickettsii organisms was analyzed by plaque formation assay using monolayers of Vero cells (39, 64). Lipopolysaccharide (LPS) was adsorbed from solutions containing R. rickettsii according to a procedure described earlier (50). In brief, aliquots of R. rickettsii were incubated with polymyxin B insolubilized on cross-linked 4% beaded agarose (binding capacity, 200 to 500 μg of LPS from Escherichia coli serotype O128:B12/ml; 10 μl/4.5 × 106 PFU of R. rickettsii). The contents of the tube were mixed by gentle tapping at 5-min intervals. After 30 min, polymyxin B agarose was allowed to settle, and supernatant was placed on the endothelial cell cultures.
Probes.
The human HO-1 and HO-2 cDNA probes were synthesized by PCR amplification of a 350-bp fragment of HO-1 (i.e., the region from bp 79 to 429) and a 1,036-bp fragment of HO-2 cDNA (region from bp −3 to 1036). The primers and conditions for PCR were adapted from those described by Kutty et al. (25, 26). The products were purified by agarose gel electrophoresis, followed by elution from the gel with a GenElute spin column. Radioactive labeling was performed by using the random primer method (Gibco-BRL, Rockville, Md.), according to the manufacturer's instructions. The HO-1 probe specifically hybridizes with ∼1.8-kb transcript from cells treated with sodium arsenite, a potent and characteristic inducer of HO-1, and the HO-2 probe recognizes both the 1.3- and 1.9-kb transcripts encoding for HO-2 in endothelial cells. The probe for human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was kindly provided by Patricia J. Simpson-Haidaris, University of Rochester, Rochester, N.Y.
Northern blot analysis.
Total RNA was extracted from cells by using TRI reagent according to the manufacturer's protocol and quantitated by measuring the UV absorbance at 260 nm. Typically, 5 to 8 μg of RNA for each condition was denatured in a glyoxal-dimethyl sulfoxide denaturing mixture and electrophoresed on a 1.0% (wt/vol) agarose in 10 mM sodium phosphate buffer (pH 7.0) with recirculation. The RNA was then transblotted to Zeta-Probe membrane (Bio-Rad, Hercules, Calif.) in 0.5× TAE (Tris-acetate-EDTA), air dried, and fixed by baking at 80°C in vacuo for 1 to 2 h. The fixed blots were prehybridized for ≥1 h, followed by hybridization at 65°C for 16 to 18 h with denatured, labeled probe added to the prehybridization buffer (ca. 0.8 × 106 to 1.2 × 106 cpm/ml). The blots were then washed, air dried, and exposed to autoradiographic film (Kodak, Rochester, N.Y.). Lane loading equivalencies were determined by hybridizing the membranes with 32P-labeled GAPDH cDNA probe. The autoradiographic images from HO-1 and HO-2 labels and the corresponding GAPDH labels were scanned with a Hewlett-Packard Scanjet 6300C scanner and quantified by using ImageQuant program (version 3.3; Molecular Dynamics, Sunnyvale, Calif.). The densities of GAPDH bands were used to correct the HO-1 and HO-2 band density values. The normalized values for control(s) were assigned a value of “1,” and the effects of infection and/or treatment were calculated as a function of this value.
Immunoblot analysis.
At the end of infection, cells were washed twice with and scraped into ice-cold phosphate-buffered saline (PBS). The cell pellet, obtained by centrifugation at 3,000 × g for 15 min at 4°C, was lysed in freshly prepared lysis buffer (1× Cell Culture Lysis Reagent [Promega, Madison, Wis.], 1 mM phenylmethylsulfonyl fluoride, 50 μg of leupeptin/ml, 100 μg of aprotinin/ml). Protein concentrations were determined by a Bio-Rad protein assay. Western blot analysis of HO-1 was done by fractionating 50 μg of protein on a 12.5% polyacrylamide gel by denaturing discontinuous gel electrophoresis according to the Laemmli method. The proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) by tank transfer (Bio-Rad). The membranes were incubated for 3 h at room temperature with a mouse anti-rat HO-1 monoclonal antibody (Stressgen, Victoria, British Columbia, Canada), diluted 1:1,000 in 10% (vol/vol) nonimmune goat serum. This was followed by incubation with peroxidase-conjugated anti-mouse immunoglobulin G (IgG) antibody (Sigma; 1:4,000 dilution) for 90 min. Labeled protein bands were examined by chemiluminescence by using SuperSignal West Dura extended duration substrate (Pierce, Rockford, Ill.). Purified recombinant HO-1 protein (5 to 10 ng) was used as a marker, and lysates of endothelial cells treated with 50 mM sodium arsenite served as a positive control.
Statistical analysis.
Data are presented as mean ± the standard error of the mean (SEM). A pooled, two-tailed t test analysis was used for the comparison of two groups of data. Results were considered statistically significant at P < 0.05.
RESULTS
R. rickettsii infection elevates HO-1 mRNA expression in endothelial cells
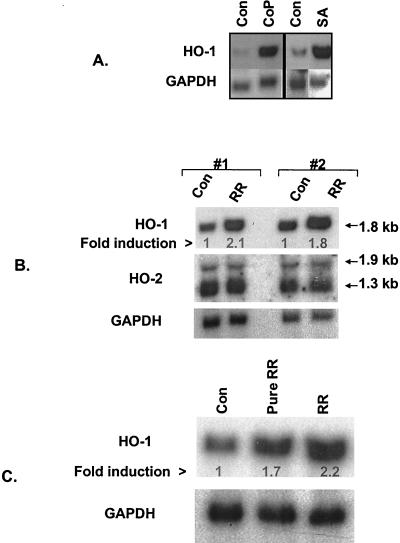
Prior to investigating the effects of R. rickettsii infection, the specificity of probes used for Northern hybridization was confirmed by analyzing the effects of known inducers of HO-1 mRNA on the expression of HO-1 and HO-2 mRNA. Endothelial cells express low basal levels of HO-1 mRNA (∼1.8-kb transcript) and incubation with cobalt protoporphyrin and sodium arsenite (50 μM for 3 h) resulted in marked induction of their HO1 mRNA levels (Fig. 1A). The HO-2 probe, on the other hand, recognized two constitutively expressed transcripts of ca. 1.9 and 1.3 kb, and neither was affected. To determine whether infection with R. rickettsii alters the expression of HO isozymes, steady-state levels of HO-1 and HO-2 mRNA were measured in cells infected for 4 h with 6 × 104 PFU/cm2 of R. rickettsii and compared with uninfected controls. As reported earlier, this regimen yielded infection of >80% of cells with four to six organisms per cell (50). A two- to threefold induction in the levels of endothelial HO-1 mRNA was observed, whereas both HO-2 transcripts remained unaffected (Fig. 1B). To ensure that HO-1 response was not due to host cell contaminates in seed stocks, endothelial cells were infected for 4 h with 1.5 × 104 PFU/cm2 of a R. rickettsii preparation that had been purified by sucrose-renografin density gradient centrifugation. This resulted in a 1.7-fold induction of HO-1 mRNA, whereas the expression of HO-2 transcripts (not shown) was unchanged (Fig. 1C).
FIG. 1.
Induction of endothelial HO-1 expression by known inducers and R. rickettsii infection. (A) Northern blot analysis of RNA from EC treated with cobalt protoporphyrin (CoP; 50 μM for 18 h) or sodium arsenite (SA; 50 μM for 3 h). Control lanes (Con) contain RNA from cells incubated with the culture medium alone. Total RNA (10 μg per lane) was electrophoresed, transferred to nylon membrane, and hybridized with a 32P-labeled human HO-1 cDNA probe. The membrane was stripped and reprobed with a human GAPDH probe to control for loading and transfer. (B) Analysis of HO-1 and HO-2 expression during R. rickettsii infection of EC. Two sets of RNA prepared from uninfected (Con) and 4-h R. rickettsii infected (RR) EC were hybridized with human HO-1, HO-2, and GAPDH probes, respectively. The positions of the transcripts for isozymes of HO are indicated by arrows. The fold induction was calculated relative to the control level for each condition, which was assigned a value of 1. (C) Comparison of HO-1 induction response in EC infected with stock versus partially purified R. rickettsii. Lane Pure RR shows HO-1 level in EC infected for 4 h with 1.5 × 104 PFU/cm2 of a sucrose-renografin density gradient-purified R. rickettsii preparation. Lane RR shows HO-1 induction obtained with a 6.0 × 104 PFU/cm2 preparation of R. rickettsii isolated as a lysate of Vero cells in the same experiment. The fold induction was calculated relative to the basal level of HO-1 expression in uninfected EC (lane 1).
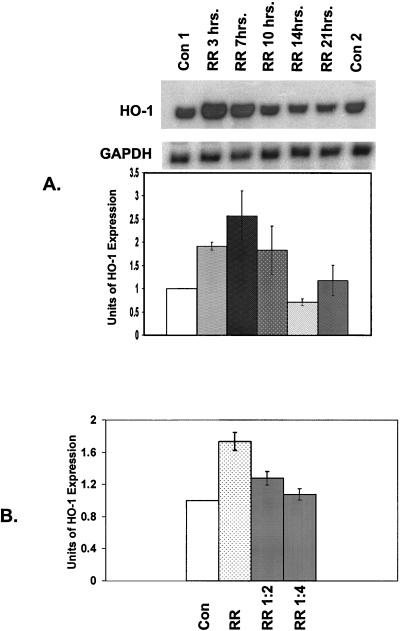
Studies were carried out to investigate whether R. rickettsii-induced HO-1 expression is dependent on the duration and dose of infection. RNA was isolated from cells infected for different times and steady-state levels of HO-1 mRNA were measured. Induction was evident at 3 h and attained a maximal level at 4 to 7 h, which was followed by a sharp decline toward the basal level (Fig. 2A). Next, endothelial cells were infected with R. rickettsii so that the inoculum ranged from 1 × 105 to 2.5 × 104 PFU/cm2. Infection-induced expression of HO-1 was proportional to the dose of infection and maximal induction occurred when cells were infected with ca. 105 PFU per cm2 of culture area (Fig. 2B). Together, these results suggest that R. rickettsii infection of endothelial cells induces the expression of HO-1 mRNA in a time- and dose-dependent manner.
FIG. 2.
(A) Time course of changes in steady-state level of HO-1 mRNA in infected EC. Total RNA was extracted from cultures of EC harvested at different times after R. rickettsii (RR) infection. Uninfected EC were used as a control (Con) and processed with both early (Con1 at 7 h) and late (Con2 at 21 h) time points. A representative Northern blot obtained with HO-1 and GAPDH probes is shown. Band intensities were quantified as described in Materials and Methods. The values for HO-1 were normalized to those for GAPDH. The basal level of HO-1 expression in uninfected cells served as control for each experiment and was assigned a value of 1. Infected conditions were then compared to corresponding controls. Data in the graph is expressed as the mean (±SEM) of three independent experiments. (B) Dependence of R. rickettsii-induced HO-1 expression on the extent (PFU/cm2) of infection. EC were infected with 105 PFU of R. rickettsii organisms per cm2 of culture area (RR), along with RR diluted 1:2 (5 × 104 PFU/cm2) and 1:4 (2.5 × 104 PFU/cm2), followed by analysis of changes in HO-1 expression level.
Induction of HO-1 mRNA by R. rickettsii requires RNA and protein synthesis.
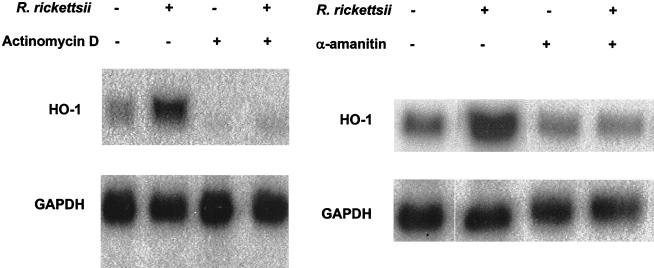
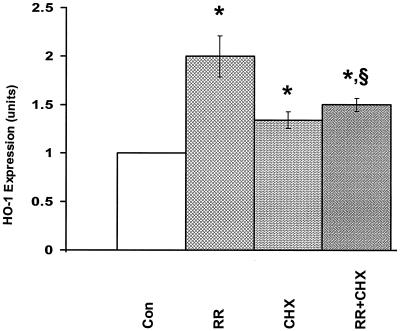
Enhancement of HO-1 expression by TNF-α and IL-1α occurs at the level of transcription in endothelial cells (54). To evaluate the mechanism of R. rickettsii-induced HO-1, cells were infected in the presence of the transcriptional inhibitors actinomycin D and α-amanitin. Both completely blocked the increase in the levels of HO-1 mRNA (Fig. 3), but the rate and extent of infection were not significantly affected. To investigate the requirement of de novo protein synthesis, endothelial cells were infected in the presence of cycloheximide. Cycloheximide treatment resulted in ca. 30% increase in the basal levels of endothelial HO-1 mRNA, and R. rickettsii-induced expression of HO-1 was almost completely abrogated (Fig. 4). These data suggest that upregulation of HO-1 expression during R. rickettsii infection of endothelial cells is dependent on both transcription and de novo protein synthesis.
FIG. 3.
Effect of eukaryotic transcriptional blockers on HO-1 mRNA induction by R. rickettsii. EC were incubated in the presence (+) or absence (−) of actinomycin D (0.25 μg/ml) and α-amanitin (5.0 μg/ml) for 30 min prior to and during a 4-h infection with R. rickettsii. Control cells were exposed to vehicle alone. Northern blots probed with a human HO-1-specific cDNA and the probe for the housekeeping gene, GAPDH, are shown. The results shown are representative of three independent experiments.
FIG. 4.
Effect of protein synthesis inhibitor cycloheximide (CHX) on EC expression of HO-1 mRNA during R. rickettsii infection. CHX (5.0 μg/ml) was added to the culture medium 30 min before and during infection for 4 h (Con = control; RR = R. rickettsii infection; CHX = cycloheximide treatment alone; RR+CHX = infection in the presence of cycloheximide). EC were processed for collection of RNA and hybridization with 32P-labeled HO-1 and GAPDH probes. Densitometric analysis of the band intensities and normalization of the data was then performed. The results are presented as mean ± the SEM of four experiments. ✽, P ≤ 0.05 in relation to Con; §, P ≥ 0.10 in comparison with CHX.
Increased endothelial expression of HO-1 mRNA requires infection with viable R. rickettsii organisms.
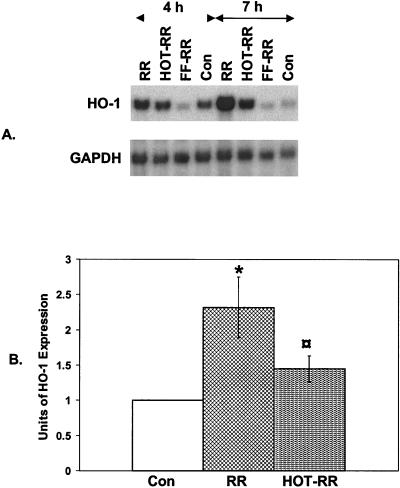
Invasion of endothelial cells by rickettsiae depends on the active participation of both organisms and endothelial cells (63). Heat- or UV-inactivated rickettsiae can bind to host cell surfaces but cannot penetrate the cells (60). To evaluate whether active rickettsia infection is a requisite for HO-1 mRNA induction, we utilized two approaches: inactivation of rickettsiae with heat or formaldehyde and inhibition of rickettsial uptake by cytochalasin B treatment of endothelial cells. Results from the plaque formation assay confirmed that both heat treatment and formalin fixation resulted in complete inhibition of infection. Next, we exposed endothelial cells for 4 and 7 h to heat-inactivated and formalin-fixed R. rickettsii, followed by analysis of HO-1 mRNA levels. Infection with inactive organisms resulted in marked reduction of HO-1 induction (Fig. 5).
FIG. 5.
Effect of heat treatment or formalin fixation of R. rickettsii on endothelial HO-1 response to infection. (A) R. rickettsii organisms were subjected to heat inactivation or formalin fixation as described in Materials and Methods. EC cultures were exposed to live (RR), heat-inactivated (HOT-RR), or formalin-fixed (FF-RR) R. rickettsii, and the abundance of HO-1 mRNA for each condition was analyzed by Northern hybridization. (B). Comparison of the levels of HO-1 expression in EC infected for 4 h with RR versus HOT-RR. Values are mean ± the SEM from three separate experiments. ✽, P ≤ 0.05 in relation to Con;  , P ≤ 0.05 in comparison with RR.
, P ≤ 0.05 in comparison with RR.
Endothelial cells were incubated with cytochalasin B (50 μM) during R. rickettsii infection to inhibit internalization of organisms, and immunofluorescence staining showed that this blocked the uptake. Northern blot analysis of RNA isolated from cells infected under these conditions revealed that R. rickettsii-induced HO-1 expression was diminished an average of 80% (n = 3), indicating that uptake of viable organisms plays an important role in HO-1 induction.
Previous studies have shown that LPS is able to induce HO-1 gene transcription in endothelial cells and macrophages (5, 32). To investigate the role of LPS in infection-induced HO-1 expression, LPS from R. rickettsii preparations was adsorbed with polymyxin B-agarose beads, which has no significant effect on the infectivity (50). Infection-induced HO-1 transcription was not affected by polymyxin B-agarose treatment, indicating lack of involvement of LPS in this response (not shown).
NAC prevents induction of endothelial HO-1 mRNA during R. rickettsii infection.
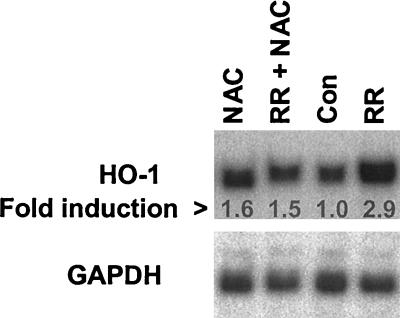
Biologically reactive oxygen species including superoxide, hydrogen peroxide, hydroxyl radicals, and lipid hydroperoxides, may function as cellular signaling entities in endothelial cells (53). Therefore, we hypothesized that generation of various reactive oxygen radicals during R. rickettsii infection may contribute to the HO-1 response. To investigate this, we utilized the antioxidant compound, NAC, which acts as a scavenger of free radicals and increases intracellular thiol levels. Cells were pretreated with 500 μM NAC for 30 min, followed by infection with R. rickettsii for 4 h. Induction of HO-1 mRNA by R. rickettsii infection was attenuated in the presence of NAC (Fig. 6), suggesting involvement of oxidative stress mechanisms in the upregulation of HO-1.
FIG. 6.
Effect of an antioxidant, NAC, on R. rickettsii-induced HO-1 mRNA expression. EC were left untreated (Con) or preincubated with NAC (500 μM) for 60 min. (NAC), followed by infection with R. rickettsii in its absence (RR) or presence (RR+NAC) for 4 h. Total RNA isolated from EC for each experimental condition was resolved by electrophoresis and the blot was hybridized with HO-1 and GAPDH probes, respectively. The results shown are representative of two independent experiments.
Analysis of HO-1 protein expression during R. rickettsii infection of endothelial cells.
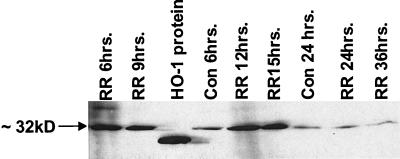
Western blot analysis was used to determine whether changes in HO-1 mRNA expression are followed by increases in protein. Cells were infected with R. rickettsii for different times, protein lysates were prepared, and immunoblotting for HO-1 was performed (Fig. 7). R. rickettsii infection caused production of an ∼32-kDa protein that was immunoreactive with HO-1-specific antibody. Higher levels of HO-1 protein appeared after 6 to 9 h, were sustained up to 12 to 15 h, and declined toward the basal level thereafter.
FIG. 7.
Western blot analysis of HO-1 protein in EC during infection with R. rickettsii. EC were left uninfected (Con) or infected for indicated lengths of time (RR), washed thoroughly with PBS, and lysed in cell lysis buffer. A total of 50 μg of sample protein were subjected to polyacrylamide gel electrophoresis. Recombinant HO-1 protein (10 ng) was used as standard. Immunoblotting was performed with a monoclonal antibody reactive with ∼32-kDa HO-1 protein. The standard protein migrates slightly lower on the gel than the native HO-1 protein because of the absence of amino acids 261 to 269 of membrane-spanning region.
DISCUSSION
HO-1 is a stress response protein that has been implicated in cytoprotective defense mechanisms against agents that induce oxidative injury, such as endotoxins, cytokines, and heavy metals. Considerable evidence now exists to support the involvement of reactive oxygen species in the endothelial cell response to infection with R. rickettsii. Whereas measures of free radical generation and lipid peroxidation are increased, there is a reduction in the levels of key enzymes responsible for protection against oxidative injury, which apparently exacerbates the stress on the infected cell (11, 47). During the state of increased oxidative stress, the host cell responds by launching an adaptive response, including increased activity of superoxide dismutase (42). In the present study, we report the effects of R. rickettsii infection on the expression of HO isozymes in endothelial cells and demonstrate increased expression of HO-1 mRNA and protein in infected endothelial cells. Under control conditions, the mRNA levels of the constitutive isoform HO-2 were higher than HO-1, but expression of both transcripts coding for HO-2 remained unaltered during infection. Consistent with the view that HO-1 is inducible and HO-2 is constitutive, these results indicate that HO-1 participates in host cell response to infection.
The increased levels of HO-1 mRNA in infected cells peaked at 4 to 7 h, and protein expression lagged by 2 to 3 h, resembling the time course of HO-1 induction by inflammatory cytokines, TNF-α and IL-1α (54). The extent of HO-1 induction by R. rickettsii is less in comparison to that observed with TNF-α and IL-1α by Terry et al. (54), or sodium arsenite, considered to be a prototypical but nonphysiological inducer of HO-1. The intracellular signaling pathways responsible for induction of HO-1 during Rickettsia infection of endothelial cells have yet to be determined. It is unlikely, however, that HO-1 induction by R rickettsii is due to the effects of TNF-α or IL-1α produced as a result of infection since no changes were evident in TNF-α mRNA expression or secretion (10), and the production of IL-1-α by infected endothelial cells follows delayed kinetics and most remains cell associated (51).
Available reports investigating the effects of many HO-1 inducers on various cell types suggest that the control of the expression of HO-1 is exerted at the level of transcription (24, 54). We also find transcriptional regulation of HO-1 in R. rickettsii-infected cells. On the other hand, the effects of inhibition of protein synthesis differ with the activating agent and cell type. Cycloheximide blocks the TNF-α- and IL-1α-mediated expression of HO-1 in endothelial cells and prostaglandin A2 induction of HO-1 in fibroblasts (6, 54), has no effects on HO-1 induction by IL-6 in hepatoma cells (30) or LPS induction of HO-1 in macrophages (5), and even causes enhanced induction of HO-1 by cobalt protoporphyrin (31). Although reported to have no effects on the level of HO-1 mRNA (35, 54), we noticed a small but statistically significant increase in the expression of HO-1 transcript after treatment of endothelial cells with cycloheximide alone. Further, R. rickettsii-induced HO-1 was suppressed by inhibition of new protein synthesis, indicating the requirement for de novo protein synthesis.
The contact of rickettsial organisms with the host cell surface may be sufficient to initiate some signaling mechanisms. Exposure of rickettsiae to formalin interferes with the ability to penetrate host cells and induce responses such as enhancement of expression of cell adhesion molecules and cytokines (12, 22). In our studies, inactivation of R. rickettsii with formaldehyde resulted in complete abrogation, whereas heat treatment caused significant inhibition of HO-1 induction. These observations were further supported by using cytochalasin B, an inhibitor which allows rickettsial interaction with the cell surface but blocks the process of invasion (52). Together, these data suggest that the HO-1 response to R. rickettsii infection depends on the intracellular uptake of viable organisms and indicate the possibility of involvement of a heat-labile rickettsial molecule. Although the properties of such a component are yet to be described, the contribution of rickettsial LPS can be excluded based on the demonstration that removal of LPS from R. rickettsii had no effect on the infection-induced HO-1 expression. As reported previously for other cell responses induced by spotted fever group rickettsiae (12, 22) and, in accordance with the obligately intracellular nature of infection, the adherence of R. rickettsii organisms to the cell surface may be insufficient to trigger HO-1 activation.
One of the common aspects of the regulation of cellular HO-1 by different types of inducers is their ability to produce oxidative radicals and, in effect, shift the ratio of oxidized versus reduced glutathione (23, 58). Based on the measures of reactive oxygen species and the evidence for altered regulation of antioxidant scavenging enzymes (11, 15, 16, 21, 42, 47), R. rickettsii-infected host cells also undergo considerable oxidative stress. Pretreatment of cells with the antioxidant compound NAC ameliorates the HO-1 response to infection, suggesting an intricate relationship between oxidant generation and upregulation of HO-1 during infection.
The molecular mechanisms underlying HO-1 induction are complex and tightly regulated at the level of transcription. The HO-1 gene contains recognition sequences for NF-κB and activator protein 1 (43). NF-κB is the first eukaryotic transcription factor shown to respond directly to oxidative stress in a variety of cells (14). We have shown that R. rickettsii infection of endothelial cells causes activation of NF-κB (9, 52). In a preliminary study to investigate the role of NF-κB activation in Rickettsia-induced HO-1 expression, we used a specific inhibitor of proteasome function, MG132. Surprisingly, incubation of cells with low doses of MG132 alone led to the upregulation of HO-1 mRNA (unpublished results). This observation is similar to the finding of induction of HO-1 with pyrrolidine dithiocarbamate, another inhibitor of NF-κB (20). Changes in the levels of intracellular calcium and activation of protein kinase C isozymes may also contribute, in part, to signaling events (55). Endothelial cell layers infected with spotted fever group and typhus group rickettsiae exhibit elevation in intracellular calcium levels leading to the activation of Ca2+-calmodulin system (J. P. Olano and G. Wen, Abstr. Am. Soc. Rickettsiol., abstr. 74, p. 55, 2001), and the involvement of protein kinase C in transcriptional activation of R. rickettsii-infected cells has been documented by our laboratory (41). Further studies to investigate the participation of these and related pathways will yield useful information on the mechanisms of R. rickettsii-induced HO-1 expression.
Endothelial cells are capable of accumulating heme, the typical HO-1 inducer, and the end products of HO reaction have crucial biological functions in vascular endothelium. Growing evidence now points to the central role of HO-1 as an inducible gene that confers cytoprotection through the generation of antioxidant bilirubin and controlling the availability of cellular prooxidant iron (34). Recent studies have implicated CO, generated through the catabolism of heme by HO-1, in the protection of endothelial cells against apoptosis (4). Furthermore, HO-1 has been recognized as an inducible protein, which mediates endothelial protection induced by a nitric oxide (NO) donor molsidomine, and the second messenger cyclic GMP (36). It has been documented that NO released from appropriately stimulated host cells is toxic to extracellular rickettsiae (59). Since HO-1 represents an important cellular target of NO donors and can serve as an “intracellular sink” for NO (17), it is plausible to speculate a physiologically relevant protective role for HO-1 in the host cell response during pathogenesis of rickettsia infection.
HO-1 has been reported to be induced as a consequence of rhinovirus type 14 infection of cultured human airway epithelial cells and found to protect against the infection and inhibit viral replication (18, 66). It was proposed that increased HO activity may also serve to limit the airway inflammation by anti-inflammatory actions (27, 33, 65), and increased CO in exhaled air of subjects with upper respiratory tract infection is attributed to induction of HO activity (67). Increased HO-1 also plays a vital role in the pathology of viral infections, such as influenza and encephalomyocarditis (8, 38), and infection with the gram-positive bacterium Listeria monocytogenes (3). To the best of our knowledge, the present study constitutes the first demonstration of induction of HO-1 at the cellular level in response to infection with an obligately intracellular bacterium. In view of the significant role of oxidants in the pathogenesis of R. rickettsii-induced cell injury, the regulation of antioxidant enzyme systems may be critical for the survival of host cell and outcome of the resultant disease. Thus, induction of HO-1 likely represents an important adaptive mechanism for moderating the severity of cell damage and modulation of inflammatory response to infection.
Acknowledgments
We thank Loel Turpin, Lisa Domotor, and Li Hua Rong for technical assistance; Dawn Clifton and Lee Ann Sporn for helpful discussions; and Charles W. Francis for critically reviewing the manuscript.
This work was supported in part by grants HL30616, AI40689, and AI17416 from the National Institutes of Health, Bethesda, Md.
Editor: E. I. Tuomanen
REFERENCES
- 1.Abraham, N. G., Y. Lavrovsky, M. L. Schwartzman, R. A. Stoltz, R. D. Levere, M. E. Gerritsen, S. Shibahara, and A. Kappas. 1995. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc. Natl. Acad. Sci. USA 92:6798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Applegate, L. A., P. Luscher, and R. Tyrell. 1991. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 51:974-978. [PubMed] [Google Scholar]
- 3.Azri, S., and K. W. Renton. 1991. Factors involved in the depression of hepatic mixed function oxidase during infections with Listeria monocytogenes. Int. J. Immunopharmacol. 13:197-204. [DOI] [PubMed] [Google Scholar]
- 4.Brouard, S., L. E. Otterbein, J. Anrather, E. Tobiasch, F. H. Bach, A. M. K. Choi, and M. P. Soares. 2000. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 192:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camhi, S. L., J. Alam, L. Otterbein, S. L. Sylvester, and A. M. Choi. 1995. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am. J. Respir. Cell Mol. Biol. 13:387-398. [DOI] [PubMed] [Google Scholar]
- 6.Choi, A. M., R. W. Tucker, S. G. Carlson, G. Weigand, and N. J. Holbrook. 1994. Calcium mediates expression of stress-response genes in prostaglandin A2-induced growth arrest. FASEB J. 8:1048-1054. [DOI] [PubMed] [Google Scholar]
- 7.Choi, A. M., and J. Alam. 1996. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 15:9-19. [DOI] [PubMed] [Google Scholar]
- 8.Choi, A. M. K., K. Knobil, S. L. Otterbein, D. A. Eastman, and D. B. Jacoby. 1996. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am. J. Physiol. 271:L383-L391. [DOI] [PubMed] [Google Scholar]
- 9.Clifton, D. R., R. A. Goss, S. K. Sahni, D. VanAntwerp, R. B. Baggs, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl. Acad. Sci. USA 95:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifton, D. R. 2000. Host cell responses to infection with the obligate intracellular parasite Rickettsia rickettsii. Ph.D. thesis. University of Rochester Medical Center, Rochester, N.Y.
- 11.Devamanoharan, P. S., L. A. Santucci, J. E. Hong, X. Tian, and D. J. Silverman. 1994. Infection of human endothelial cells by Rickettsia rickettsii causes a significant reduction in the levels of key enzymes involved in protection against oxidative injury. Infect. Immun. 62:2619-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dignat-George, F., N. Teysseire, M. Mutin, N. Bardin, G. Lesaule, D. Raoult, and J. Sampol. 1997. Rickettsia conorii infection enhances vascular cell adhesion molecule-1- and intercellular adhesion molecule-1-dependent mononuclear cell adherence to endothelial cells. J. Infect. Dis. 175:1142-1152. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt, M., M. C. Alessi, P. Y. Levy, I. Juhan-Vague, and D. Raoult. 1990. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by Rickettsia conorii- and Rickettsia rickettsii-infected cultured endothelial cells. Infect. Immun. 58:2459-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dröge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47-95. [DOI] [PubMed] [Google Scholar]
- 15.Eremeeva, M. E., and D. J. Silverman. 1998. Effects of the antioxidant α-lipoic acid on human umbilical vein endothelial cells infected with Rickettsia rickettsii. Infect. Immun. 66:2290-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eremeeva, M. E., and D. J. Silverman. 1998. Rickettsia rickettsii infection of the EA.hy 926 endothelial cell line: morphological response to infection and evidence for oxidative injury. Microbiology 144:2037-2048. [DOI] [PubMed] [Google Scholar]
- 17.Foresti, R., J. E. Clark, C. J. Green, and R. Motterlini. 1997. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J. Biol. Chem. 272:18411-18417. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima, T., S. Okinaga, K. Sekizawa, T. Ohrui, M. Yamaya, H. Sasaki. 1995. The role of carbon monoxide in lucigenin-dependent chemiluminescence of rat alveolar macrophages. Eur. J. Pharmacol. 289:103-107. [DOI] [PubMed] [Google Scholar]
- 19.Gimbrone, M. A., Jr., R. S. Cotran, and J. Folkman. 1974. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 60:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartsfield, C. L., J. Alam, and A. M. Choi. 1998. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 12:1675-1682. [DOI] [PubMed] [Google Scholar]
- 21.Hong, J. E., L. A. Santucci, X. Tian, and D. J. Silverman. 1998. Superoxide dismutase-dependent, catalase-sensitive peroxides in human endothelial cells infected by Rickettsia rickettsii. Infect. Immun. 66:1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplanski, G., N. Teysseire, C. Farnarier, S. Kaplanski, J.-C. Lissitzky, J.-M. Durand, J. Soubeyrand, C. A. Dinarello, and P. Bongrand. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1α-dependent pathway. J. Clin. Investig. 96:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keyse, S. M., and R. M. Tyrrell. 1989. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA 86:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyse, S. M., L. A. Applegate, Y. Tromvoukis, and R. M. Tyrrell. 1990. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol. Cell. Biol. 10:4967-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutty, G., B. Hayden, Y. Osawa, B. Wiggert, G. J. Chader, and R. K. Kutty. 1992. Heme oxygenase: expression in human retina and modulation by stress agents in a human retinoblastoma cell model system. Current Eye Res. 11:153-160. [DOI] [PubMed] [Google Scholar]
- 26.Kutty, R. K., G. Kutty, C. N. Nagineni, J. J. Hooks, G. J. Chader, and B. Wiggert. 1994. RT-PCR assay for heme oxygenase-1 and heme oxygenase-2: a sensitive method to estimate cellular oxidative damage. Ann. N. Y. Acad. Sci. 738:427-430. [DOI] [PubMed] [Google Scholar]
- 27.Lee, P. J., J. Alam, G. W. Wiegand, and A. M. Choi. 1996. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA 93:10393-10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines, M.D. 1997. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37:517-554. [DOI] [PubMed] [Google Scholar]
- 29.McCoubrey, W. K., Jr., T. J. Huang, and M. D. Maines. 1997. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 247:725-732. [DOI] [PubMed] [Google Scholar]
- 30.Mitani, K., H. Fujita, A. Kappas, and S. Sassa. 1992. Heme oxygenase is a positive acute-phase reactant in human Hep3B hepatoma cells. Blood 79:1255-1259. [PubMed] [Google Scholar]
- 31.Mitani, K., H. Fujita, Y. Fukuda, A. Kappas, and S. Sassa. 1993. The role of inorganic metals and metalloporphyrins in the induction of haem oxygenase and heat-shock protein 70 in human hepatoma cells. Biochem. J. 290:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motterlini, R., R. Foresti, M. Intaglietta, and R. M. Winslow. 1996. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am. J. Physiol. 270:H107-H114. [DOI] [PubMed] [Google Scholar]
- 33.Nath, K. A., G. Balla, G. M. Vercellotti, J. Balla, H. S. Jacob, M. D. Levitt, and M. E. Rosenberg. 1992. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 90:267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otterbein, L. E., and A. M. K. Choi. 2000. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1029-L1037. [DOI] [PubMed] [Google Scholar]
- 35.Panchenko, M. V., H. W. Farber, and J. H. Korn. 2000. Induction of heme oxygenase-1 by hypoxia and free radicals in human dermal fibroblasts. Am. J. Physiol. Cell Physiol. 278:C92-C101. [DOI] [PubMed] [Google Scholar]
- 36.Polte, T., A. Abate, P. A. Dennery, and H. Schröder. 2000. Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. Arterioscler. Thromb. Vasc. Biol. 20:1209-1215. [DOI] [PubMed] [Google Scholar]
- 37.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renton, K. W. 1981. Depression of hepatic cytochrome P-450-dependent mixed function oxidases during infection with encephalomyocarditis virus. Biochem. Pharmacol. 30:2333-2336. [DOI] [PubMed] [Google Scholar]
- 39.Rolain, J. M., M. Maurin, G. Vestris, and D. Raoult. 1998. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob. Agents Chemother. 42:1537-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahni, S. K., D. J. VanAntwerp, M. E. Eremeeva, D. J. Silverman, V. J. Marder, and L. A. Sporn. 1998. Proteasome-independent activation of nuclear factor κB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect. Immun. 66:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahni, S. K., L. C. Turpin, T. L. Brown, and L. A. Sporn. 1999. Involvement of protein kinase C in Rickettsia rickettsii-induced transcriptional activation of the host endothelial cell. Infect. Immun. 67:6418-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santucci, L. A., P. L. Gutierrez, and D. J. Silverman. 1992. Rickettsia rickettsii induces superoxide radical and superoxide dismutase in human endothelial cells. Infect. Immun. 60:5113-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen, C. K., and L. Packer. 1996. Antioxidant and redox regulation of gene transcription. FASEB J. 10:709-720. [DOI] [PubMed] [Google Scholar]
- 44.Shi, R.-J., P. J. Simpson-Haidaris, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1996. Increased expression of plasminogen activator inhibitor-1 in R. rickettsii-infected endothelial cells. Thromb. Haemost. 75:600-606. [PubMed] [Google Scholar]
- 45.Silverman, D. J. 1984. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect. Immun. 44:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman, D. J., and S. B. Bond. 1984. Infection of human vascular endothelial cells by Rickettsia rickettsii. J. Infect. Dis. 149:201-206. [DOI] [PubMed] [Google Scholar]
- 47.Silverman, D. J., and L. A. Santucci. 1988. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect. Immun. 56:3110-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman, D. J., and L. A. Santucci. 1990. A potential protective role for thiols against cell injury caused by Rickettsia rickettsii. Ann. N. Y. Acad. Sci. 590:111-117. [DOI] [PubMed] [Google Scholar]
- 49.Sporn, L. A., S. O. Lawrence, D. J. Silverman, and V. J. Marder. 1993. E-selectin-dependent neuutrophil adhesion to Rickettsia rickettsii-infected endothelial cells. Blood 81:2406-2412. [PubMed] [Google Scholar]
- 50.Sporn, L. A., P. J. Haidaris, R.-J. Shi, Y. Nemerson, D. J. Silverman, and V. J. Marder. 1994. Rickettsia rickettsii infection of cultured human endothelial cells induces tissue factor expression. Blood 83:1527-1534. [PubMed] [Google Scholar]
- 51.Sporn, L. A., and V. J. Marder. 1996. Interleukin-1α production during Rickettsia rickettsii infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect. Immun. 64:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sporn, L. A., S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab. 1997. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect. Immun. 65:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, Y. J., H. J. Forman, and A. Sevanian. 1997. Oxidants as stimulators of signal transduction. Free Radical Biol. Med. 22:269-285. [DOI] [PubMed] [Google Scholar]
- 54.Terry, C. M., J. A. Clikeman, J. R. Hoidal, and K. S. Callahan. 1998. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. 274:H883-H891. [DOI] [PubMed] [Google Scholar]
- 55.Terry, C. M., J. A. Clikeman, J. R. Hoidal, and K. S. Callahan. 1999. TNF-α and IL-1α induce heme oxygenase-1 via protein kinase C, Ca2+, and phospholipase A2 in endothelial cells. Am. J. Physiol. 276:H1493-H1501. [DOI] [PubMed] [Google Scholar]
- 56.Teysseire, N., D. Arnoux, F. George, J. Sampol, and D. Raoult. 1992. von Willebrand factor release and thrombomodulin and tissue factor expression in Rickettsia conorii-infected endothelial cells. Infect. Immun. 60:4388-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorner, A. R., D. H. Walker, and W. A. Petri, Jr. 1998. Rocky Mountain spotted fever. Clin. Infect. Dis. 27:1353-1360. [DOI] [PubMed] [Google Scholar]
- 58.Tomaro, M. L., J. Frydman, and R. B. Frydman. 1991. Heme oxygenase induction by CoCl2, Co-protoprophyrin IX, phenylhydrazine, and diamide: evidence for oxidative stress involvement. Arch. Biochem. Biophys. 286:610-617. [DOI] [PubMed] [Google Scholar]
- 59.Turco, J., H. Liu, S. F. Gottlieb, and H. H. Winkler. 1998. Nitric oxide-mediated inhibition of the ability of Rickettsia prowazekii to infect mouse fibroblasts and mouse macrophagelike cells. Infect. Immun. 66:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urakami, H., T. Tsuruhara, and A. Tamura. 1983. Penetration of Rickettsia tsutsugamushi into cultured mouse fibroblasts (L cells): an electron microscopic observation. Microbiol. Immunol. 27:251-263. [DOI] [PubMed] [Google Scholar]
- 61.Wagner, D. D., J. B. Olmsted, and V. J. Marder. 1982. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J. Cell Biol. 95:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker, D. H. 1998. Tick-transmitted infectious diseases in the United States. Annu. Rev. Public Health 19:237-269. [DOI] [PubMed] [Google Scholar]
- 63.Walker, T. S. 1984. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect. Immun. 44:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wike, D. A., and W. Burgdorfer. 1972. Plaque formation in tissue cultures by Rickettsia rickettsii isolated directly from whole blood and tick hemolymph. Infect. Immun. 6:736-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willis, D., A. R. Moore, R. Frederick, and D. A. Willoughby. 1996. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 2:87-90. [DOI] [PubMed] [Google Scholar]
- 66.Yamada, N., M. Yamaya, S. Okinaga, M. Terajima, R. Lee, T. Suzuki, K. Sekizawa, H. Suzuki, and H. Sasaki. 1997. Heme oxygenase 1 inhibits rhinovirus type 14 (HRV-14) infection and replication by cultured human trachael epithelium. Am. J. Respir. Crit. Care Med. 155:A943. [DOI] [PubMed] [Google Scholar]
- 67.Yamaya, M., K. Sekizawa, S. Ishizuka, M. Monma, K. Mizuta, and H. Sasaki. 1998. Increased carbon monoxide in exhaled air of subjects with upper respiratory tract infections. Am. J. Respir. Crit. Care Med. 158:311-314. [DOI] [PubMed] [Google Scholar]