Abstract
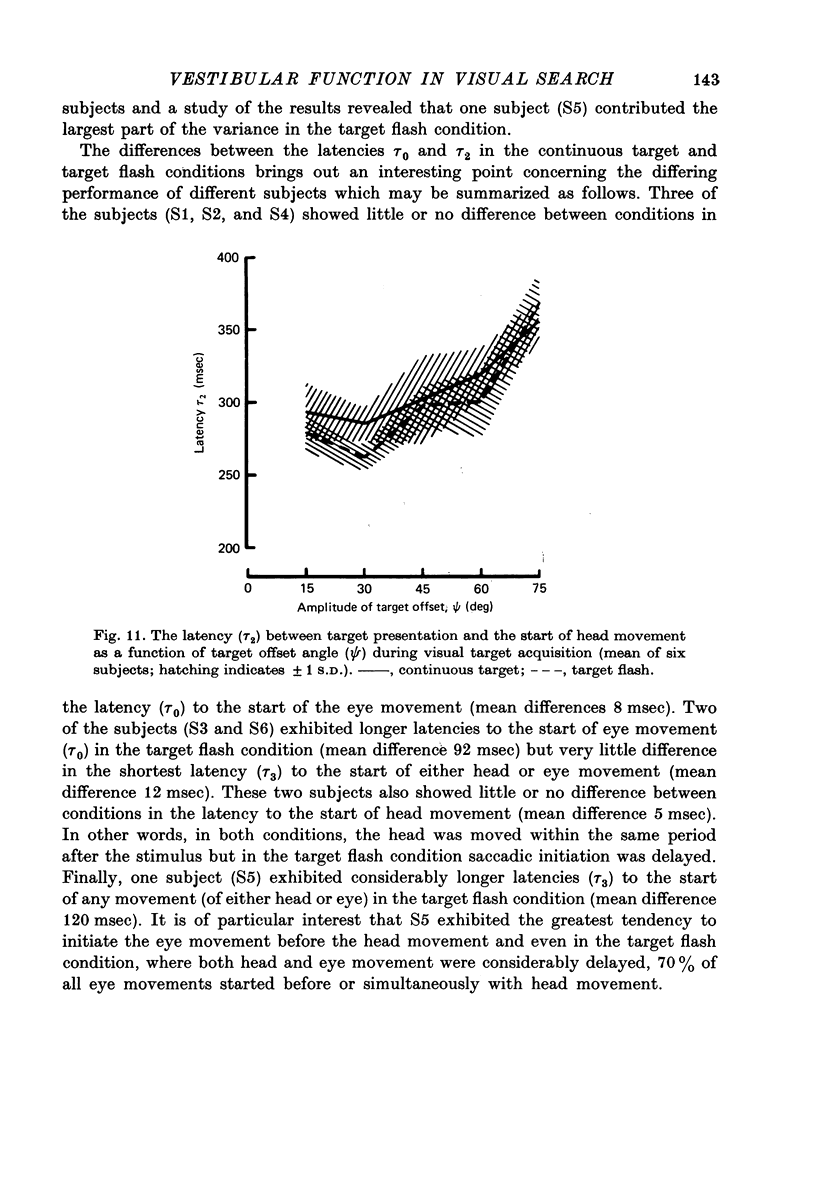
1. Experiments have been conducted on human subjects in an attempt to establish the role of the vestibulo-ocular reflex in the co-ordination of head and eye movements during visual target acquisition. 2. When the subject moved head and eyes to acquire visual targets in the horizontal plane, the eye movement consisted of an initial saccade in the direction of head movement followed by a slower return towards orbital centre which compensated for remaining head movement. 3. When the head was moved either voluntarily or passively in the dark the pattern of eye movement was very similar to that seen during target acquisition. 4. The mean latency between the start of head acceleration and the onset of the saccadic eye movement was greater in the dark (108 msec, S.D. 85 msec) than for the visually induced responses (14 msec, S.D. 59 msec), in which eye movement often preceded head movement when moving to small ( less than 45 degrees) target offset angles. 5. In all experimental conditions gaze displacement at the end of the initial saccade was normally related in a predictive manner to final head position, but when fixating visual targets offset by more than 60 degrees from the central position there were often large errors, 22% of responses undershooting the target by more than 15 degrees. 6. A highly significant (P less than 0.001) linear relationship was found between gaze displacement and head velocity under all experimental conditions. During target acquisition head velocity was normally positively correlated with amplitude of target offset. The large errors in gaze displacement in response to the larger target offsets occurred at levels of head velocity lower than normally associated with such target offsets. 7. The results have led to the suggestion of a dual mode of control for head-eye co-ordination. In one mode, normally associated with small target offsets (less than 45 degrees), control is mediated by retinal error information. In the other mode, associated with larger target offsets, gaze displacement is generated as an automatic response to head turning. 8. The observation of similar relationships between head and eye movement during passive head turning implicates the vestibulo-ocular reflex in the secondary mode of control, and provides support for the hypothesis that the role of the vestibular saccade is to induce a rapid offset of the eyes in the direction of head movement, thus facilitating rapid search and target location.
Full text
PDF



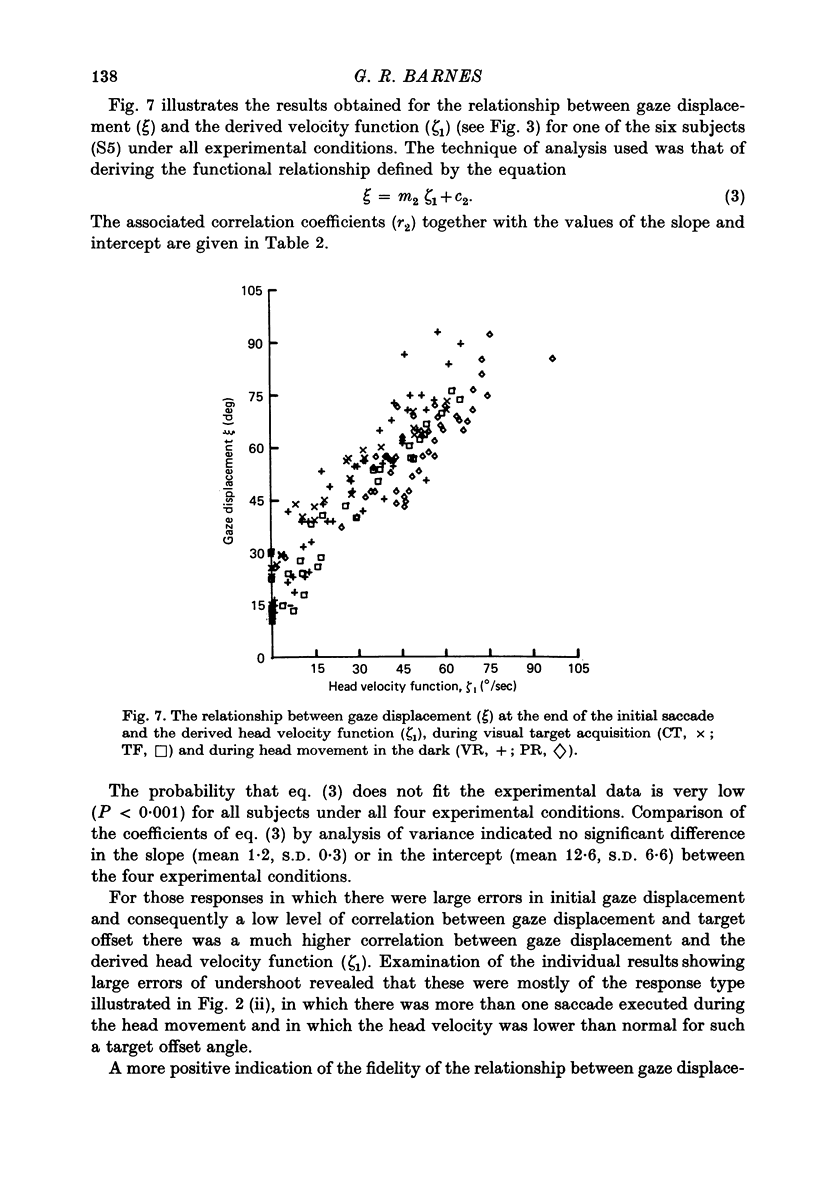
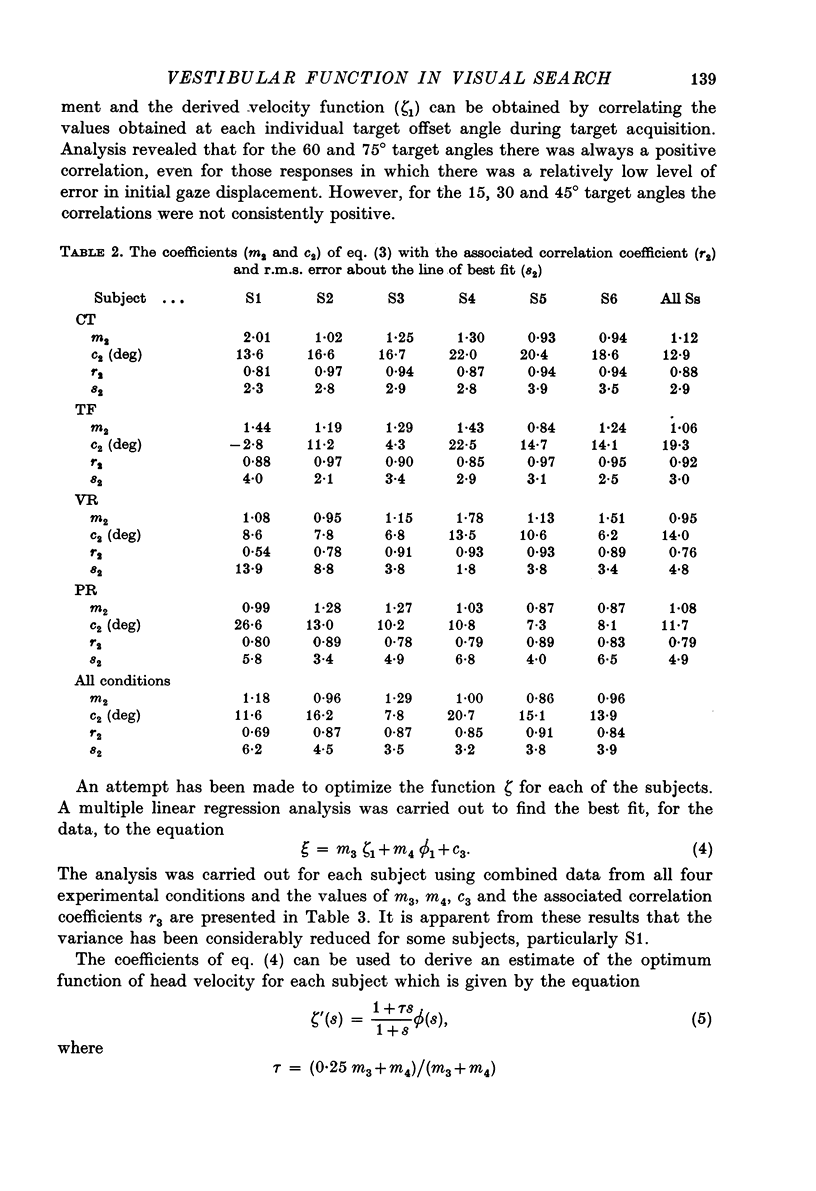
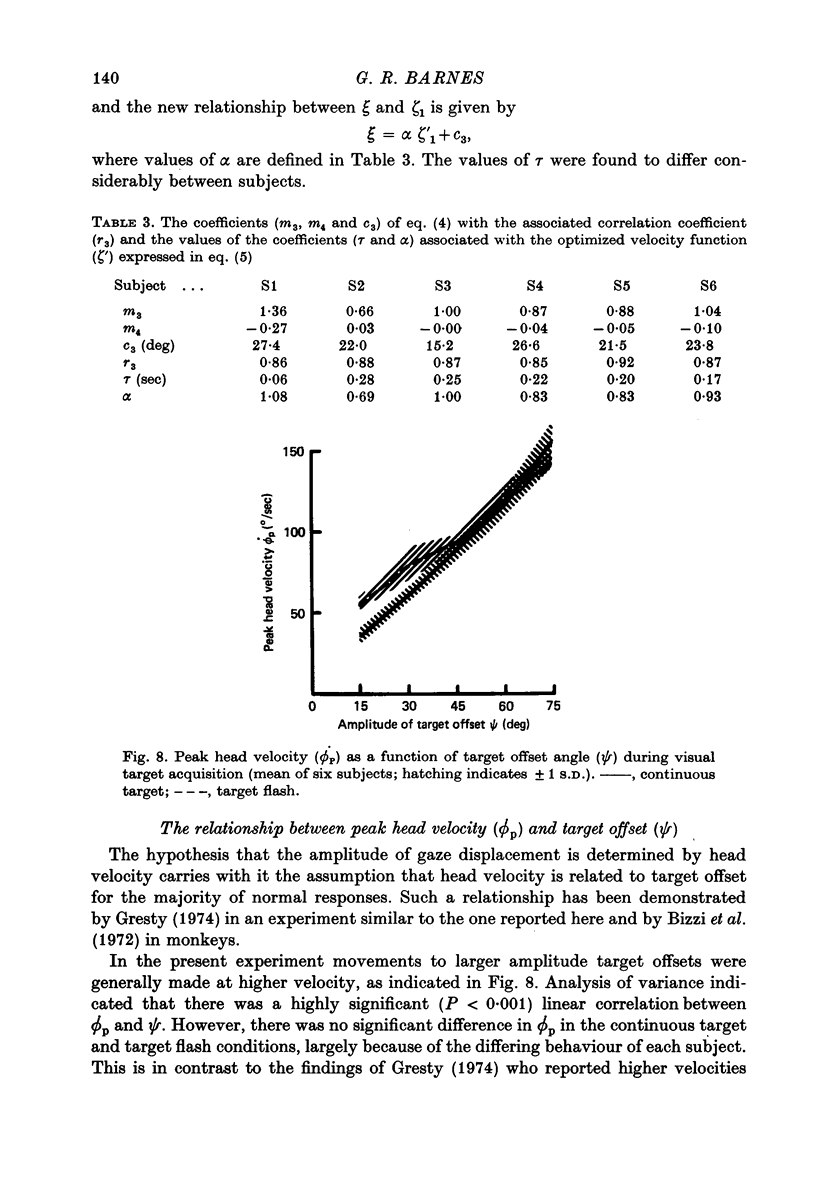

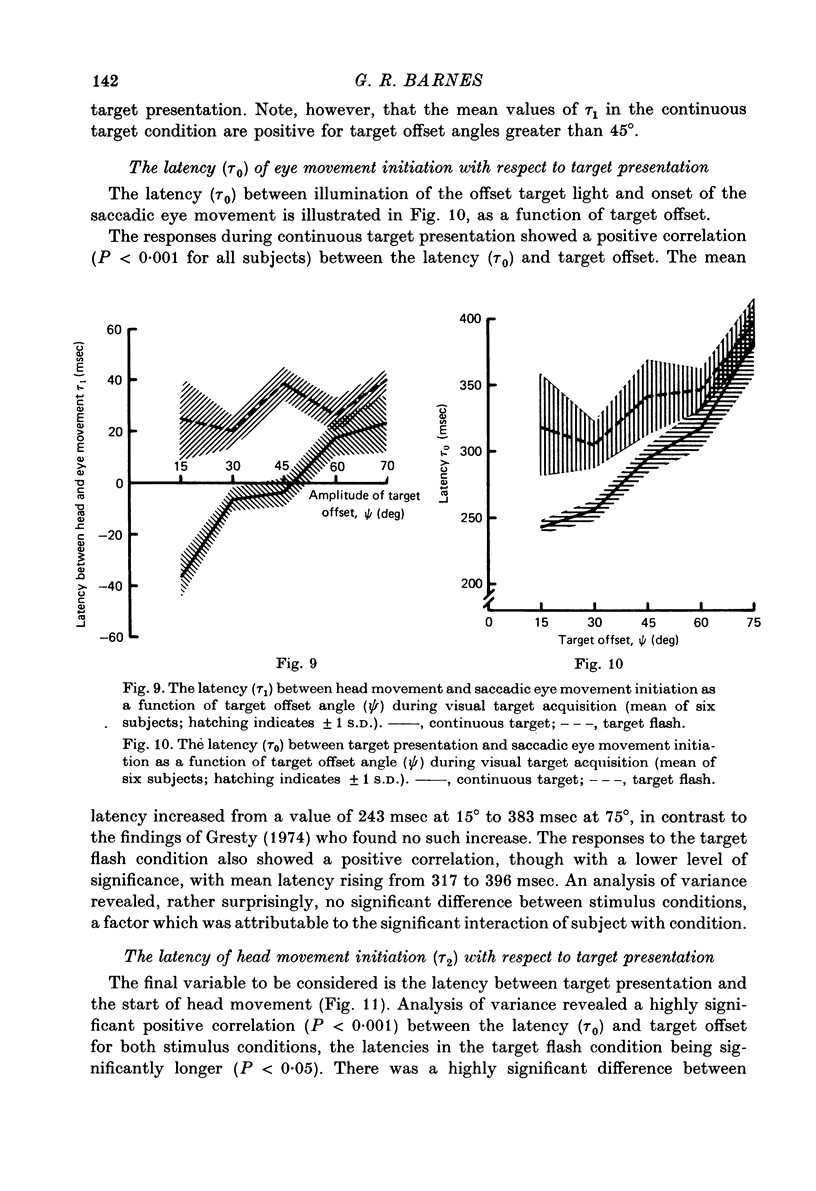




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Yoshida M., Wilson V. J. Influence of superior colliculus on cat neck motoneurons. J Neurophysiol. 1971 Sep;34(5):898–907. doi: 10.1152/jn.1971.34.5.898. [DOI] [PubMed] [Google Scholar]
- Barnes G. R., Benson A. J., Prior A. R. Visual-vestibular interaction in the control of eye movement. Aviat Space Environ Med. 1978 Apr;49(4):557–564. [PubMed] [Google Scholar]
- Barnes G. R., Sommerville G. P. Visual target acquisition and tracking performance using a helmet-mounted sight. Aviat Space Environ Med. 1978 Apr;49(4):565–572. [PubMed] [Google Scholar]
- Bartz A. E. Eye and head movements in peripheral vision: nature of compensatory eye movements. Science. 1966 Jun 17;152(3729):1644–1645. doi: 10.1126/science.152.3729.1644. [DOI] [PubMed] [Google Scholar]
- Benson A. J., Barnes G. R. Vision during angular oscillation: the dynamic interaction of visual and vestibular mechanisms. Aviat Space Environ Med. 1978 Jan;49(1 Pt 2):340–345. [PubMed] [Google Scholar]
- Bizzi E., Kalil R. E., Morasso P., Tagliasco V. Central programming and peripheral feedback during eye-head coordination in monkeys. Bibl Ophthalmol. 1972;82:220–232. [PubMed] [Google Scholar]
- FAULKNER R. F., HYDE J. E. Coordinated eye and body movements evoked by brainstem stimulation in decerebrated cats. J Neurophysiol. 1958 Mar;21(2):171–182. doi: 10.1152/jn.1958.21.2.171. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Wurtz R. H. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J Neurophysiol. 1972 Jul;35(4):542–559. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- Gonshor A., Malcolm R. Effect of changes in illumination level on electro-oculography (EOG). Aerosp Med. 1971 Feb;42(2):138–140. [PubMed] [Google Scholar]
- Gresty M. A. Coordination of head and eye movements to fixate continuous and intermittent targets. Vision Res. 1974 Jun;14(6):395–403. doi: 10.1016/0042-6989(74)90238-7. [DOI] [PubMed] [Google Scholar]
- JONES G. M. PREDOMINANCE OF ANTI-COMPENSATORY OCULOMOTOR RESPONSE DURING RAPID HEAD ROTATION. Aerosp Med. 1964 Oct;35:965–968. [PubMed] [Google Scholar]
- McIlwain J. T. Topographic relationships in projection from striate cortex to superior colliculus of the cat. J Neurophysiol. 1973 Jul;36(4):690–701. doi: 10.1152/jn.1973.36.4.690. [DOI] [PubMed] [Google Scholar]