Abstract
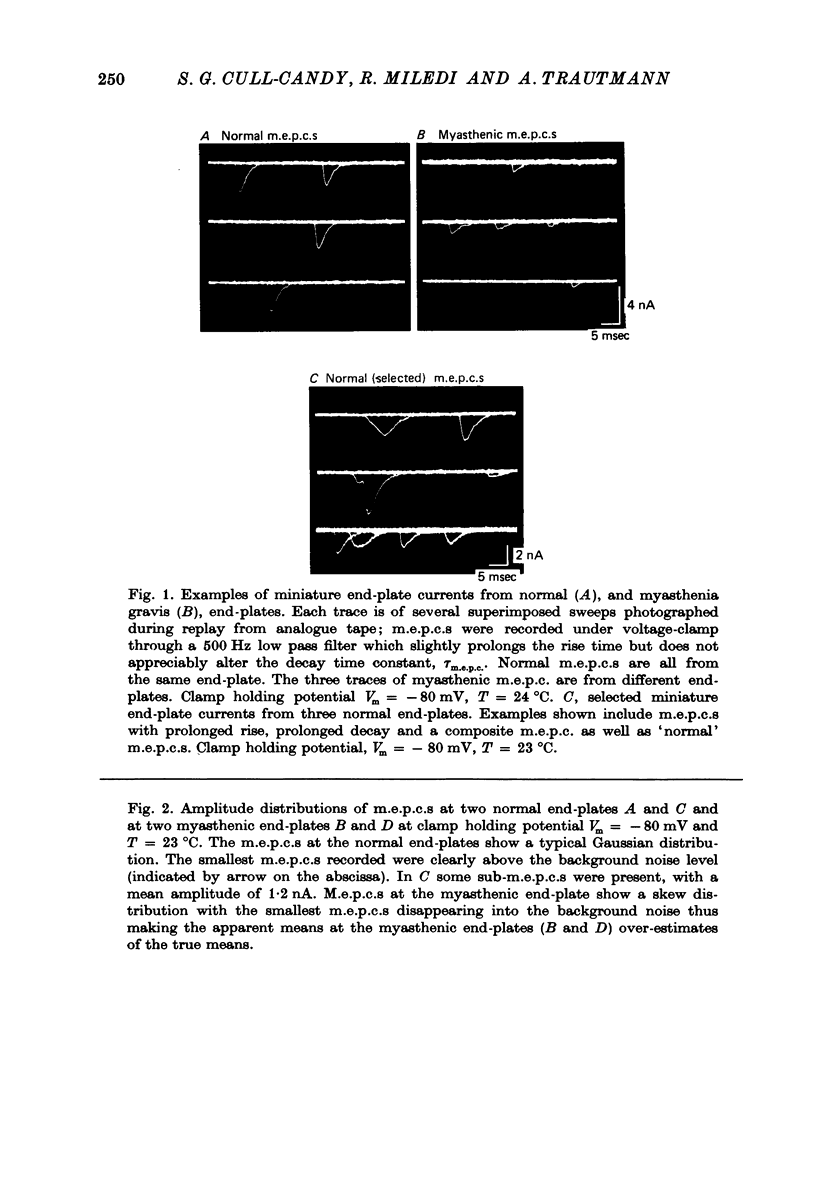
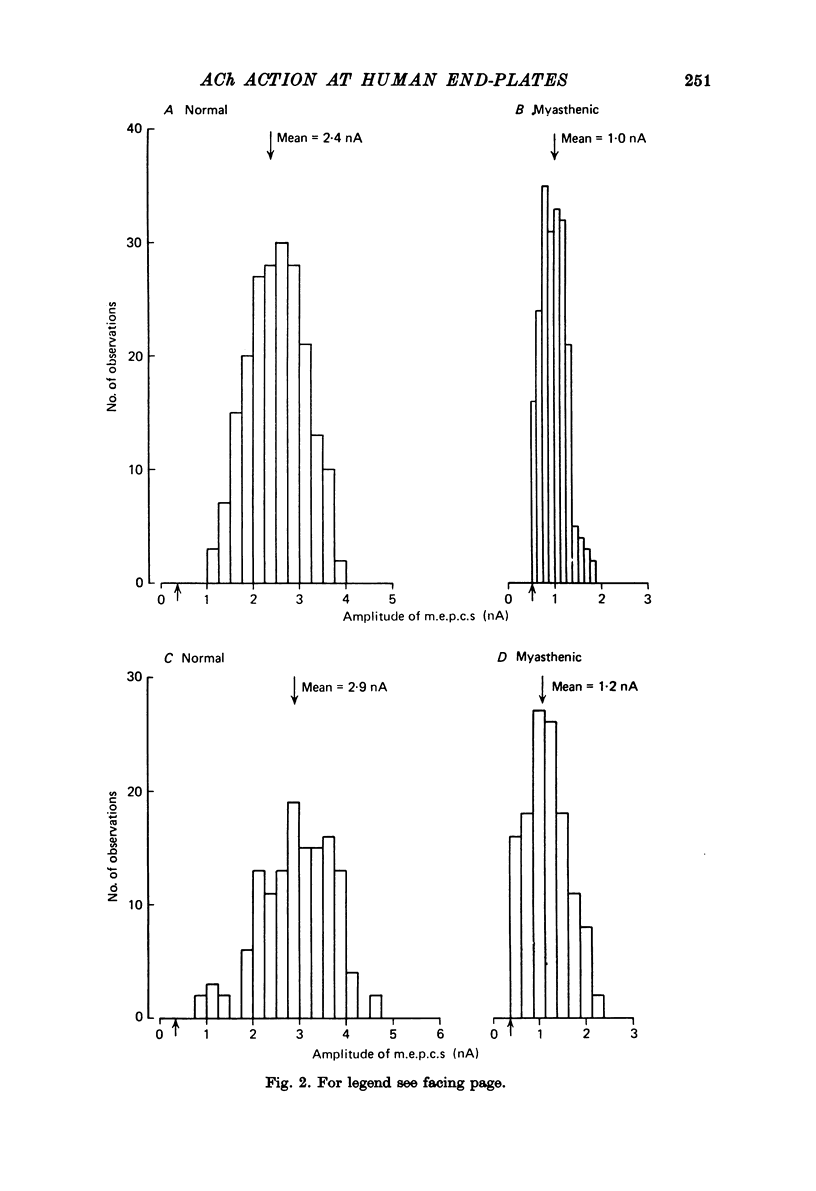
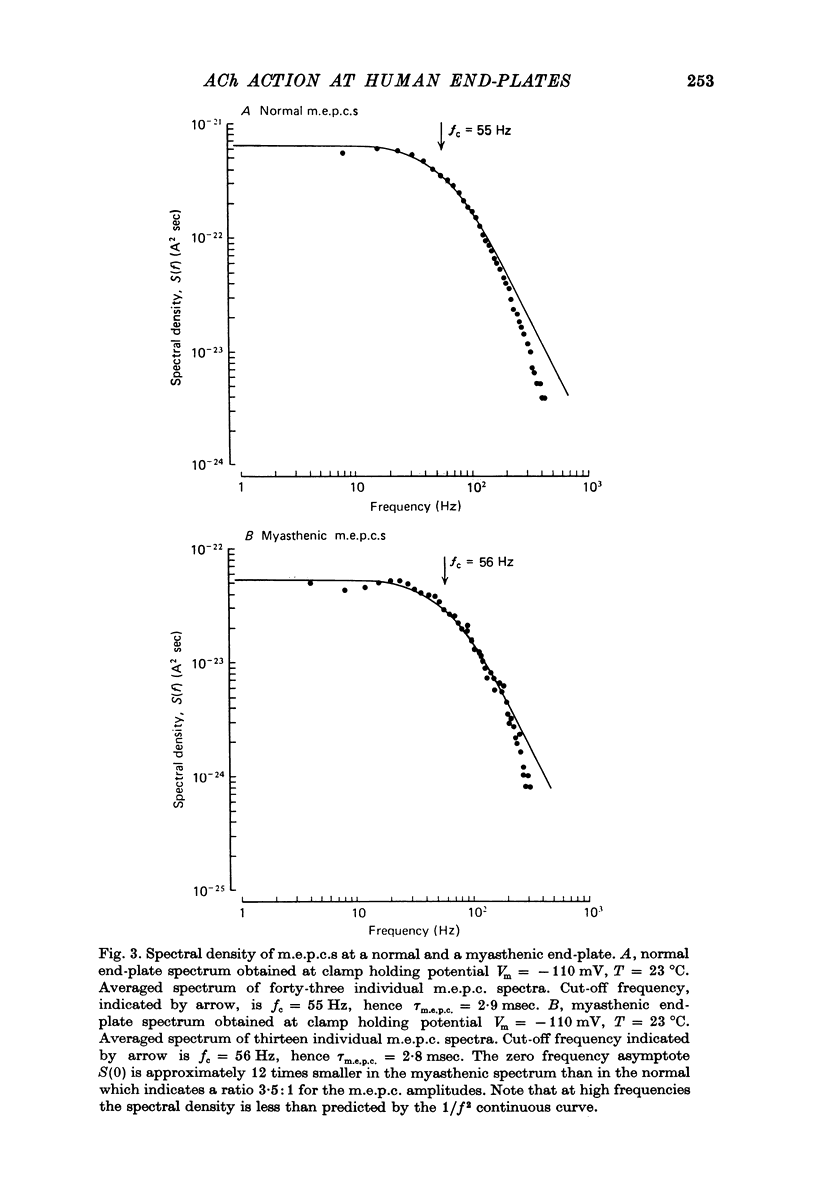
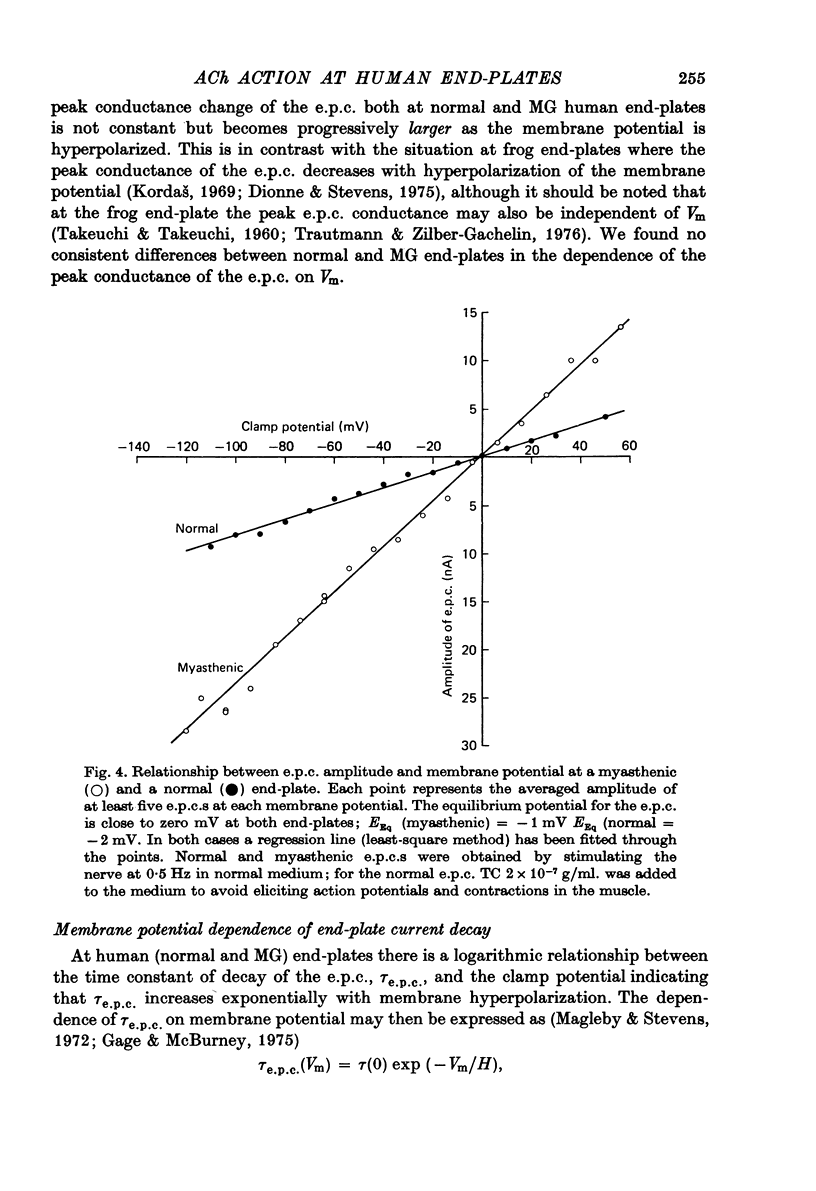
1. The amplitudes and time courses of miniature end-plate currents (m.e.p.c.s) have been compared at normal and myasthenic (MG) human end-plates studied under voltage clamp. The m.e.p.c. amplitude at MG end-plates is reduced to about one third normal; mean m.e.p.c. (normal) = 2.6 +/- 0.2 nA, mean m.e.p.c. (MG) = 1.0 +/- 0.1 nA. The decay time constant of m.e.p.c.s (tau m.e.p.c.) is very similar at normal and MG end-plates; tau m.e.p.c. (normal) = 1.70 +/- 0.1 msec, tau m.e.p.c. (MG) = 1.80 +/- 0.13 msec (Vm = - 80 mV. T = 23 degrees C). 2. The equilibrium potential of the end-plate current (e.p.c.) at normal and myasthenic human end-plates is close to 0 mV. 3. Decay time constants tau e.p.c. and tau m.e.p.c. increase exponentially with membrane hyperpolarization. The voltage sensitivity of the time constants was similar at normal and MG end-plates. 4. Both normal and myasthenic e.p.c.s are greatly prolonged in the presence of neostigmine (10(-6) g/ml.). At the same time the voltage sensitivity of tau e.p.c. is slightly reduced. 5. In response to steady ionophoretically applied ACh the mean membrane currents obtained at MG end-plates were smaller than the normal under similar conditions. 6. Analysis of end-plate current noise obtained during the steady application of acetylcholine (ACh) to voltage clamped normal and MG human end-plates showed that the amplitude of the elementary current event (gamma) and the average channel life-fime (tau noise) was similar at the two sites: tau noise (normal) - 1.54 +/- 0.04 msec, tau noise (MG) = 1.60 +/- 0.11 msec; gamma(normal) - 22.3 +/- 1.57 PS, gamma (MG) = 20.25 +/- 1.93 pS (Vm = - 80 mV, T = 23 degrees C). The voltage sensitivity of the channel life time, measured from end-plate current noise, was similar at normal and MG end-plates. 7. At normal human end-plates a packet of transmitter opens about 1500 channels whereas at MG end-plates a packet opens only about 600 channels. It is calculated that the size of the transmitter packets released from MG-terminals is at least as large as the packet of the ACh released from normal human nerve terminals.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Rash J. E., Mayer R. F., Satterfield J. R. An electrophysiological and morphological study of the neuromuscular junction in patients with myasthenia gravis. Exp Neurol. 1976 Jun;51(3):536–563. doi: 10.1016/0014-4886(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Almon R. R., Andrew C. G., Appel S. H. Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science. 1974 Oct 4;186(4158):55–57. doi: 10.1126/science.186.4158.55. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Appel S. M., Narahashi T. Myasthenia gravis serum reduces acetylcholine sensitivity in cultured rat myotubes. Nature. 1977 May 19;267(5608):262–263. doi: 10.1038/267262a0. [DOI] [PubMed] [Google Scholar]
- Bevan S., Kullberg R. W., Heinemann S. F. Human myasthenic sera reduce acetylcholine sensitivity of human muscle cells in tissue culture. Nature. 1977 May 19;267(5608):263–265. doi: 10.1038/267263a0. [DOI] [PubMed] [Google Scholar]
- Bevan S. Sub-miniature end-plate potentials at untreated frog neuromuscular junctions. J Physiol. 1976 Jun;258(1):145–155. doi: 10.1113/jphysiol.1976.sp011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coërs C., Telerman-Toppet N. Morphological and histochemical changes of motor units in myasthenia. Ann N Y Acad Sci. 1976;274:6–19. doi: 10.1111/j.1749-6632.1976.tb47672.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. Acetylcholine-induced channels and transmitter release at human endplates. Nature. 1978 Jan 5;271(5640):74–75. doi: 10.1038/271074a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., HOFMANN W. W., KUGELBERG J., QUASTEL D. M. AN ELECTROPHYSIOLOGICAL INVESTIGATION OF NEUROMUSCULAR TRANSMISSION IN MYASTHENIA GRAVIS. J Physiol. 1964 Nov;174:417–434. doi: 10.1113/jphysiol.1964.sp007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Howard F. M. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977 May;52(5):267–280. [PubMed] [Google Scholar]
- Engel A. G., Santa T. Histometric analysis of the ultrastructure of the neuromuscular junction in myasthenia gravis and in the myasthenic syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:46–63. doi: 10.1111/j.1749-6632.1971.tb30741.x. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Tsujihata M., Lindstrom J. M., Lennon V. A. The motor end plate in myasthenia gravis and in experimental autoimmune myasthenia gravis. A quantitative ultrastructural study. Ann N Y Acad Sci. 1976;274:60–79. doi: 10.1111/j.1749-6632.1976.tb47676.x. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. P., Miledi R., Perez de la Mora M., Vincent A. Acetylcholine receptors. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):551–559. doi: 10.1098/rstb.1975.0031. [DOI] [PubMed] [Google Scholar]
- Ito Y., Miledi R., Molenaar P. C., Vincent A., Polak R. L., van Gelder M., Davis J. N. Acetylcholine in human muscle. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):475–480. doi: 10.1098/rspb.1976.0025. [DOI] [PubMed] [Google Scholar]
- Ito Y., Miledi R., Vincent A., Newsom-Davis J. Acetylcholine receptors and end-plate electrophysiology in myasthenia gravis. Brain. 1978 Jun;101(2):345–368. doi: 10.1093/brain/101.2.345. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The reversal potential at the desensitized endplate. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):329–334. doi: 10.1098/rspb.1977.0144. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Potapova T. V. Potentsialy ravnovesiia vnesinapticheskoi membrany denervirovannoi myshtsy pri izmenenii vnekletochnoi ionnoi sredy. Biofizika. 1969 Jul-Aug;14(4):658–662. [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Albuquerque E. X., Hudson C. S., Mayer R. F., Satterfield J. R. Studies of human myasthenia gravis: electrophysiological and ultrastructural evidence compatible with antibody attachment to acetylcholine receptor complex. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4584–4588. doi: 10.1073/pnas.73.12.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa T., Engel A. G., Lambert E. H. Histometric study of neuromuscular junction ultrastructure. I. Myasthenia gravis. Neurology. 1972 Jan;22(1):71–82. doi: 10.1212/wnl.22.1.71. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A., Zilber-Gachelin N. F. Further investigations on the effect of denervation and pH on the conductance change at the neuromuscular junction of the frog. Pflugers Arch. 1976 Jun 29;364(1):53–58. doi: 10.1007/BF01062911. [DOI] [PubMed] [Google Scholar]


