Abstract
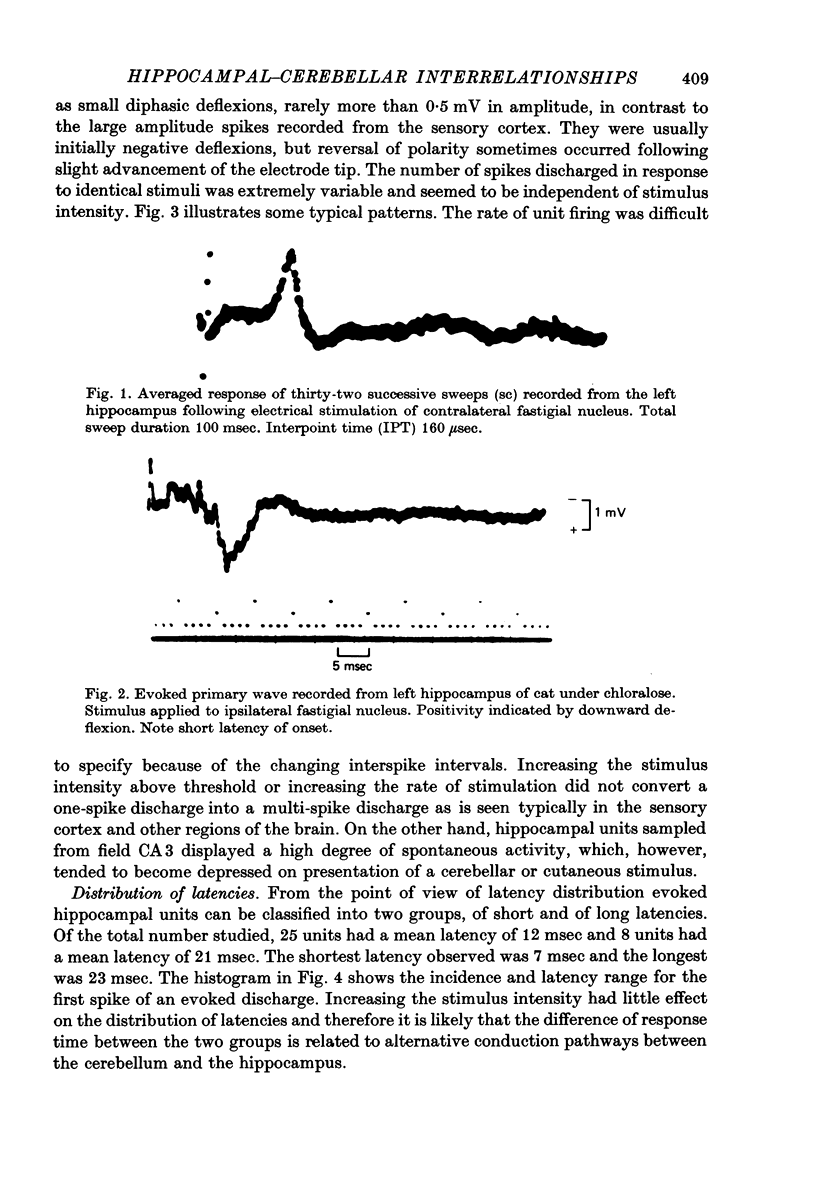
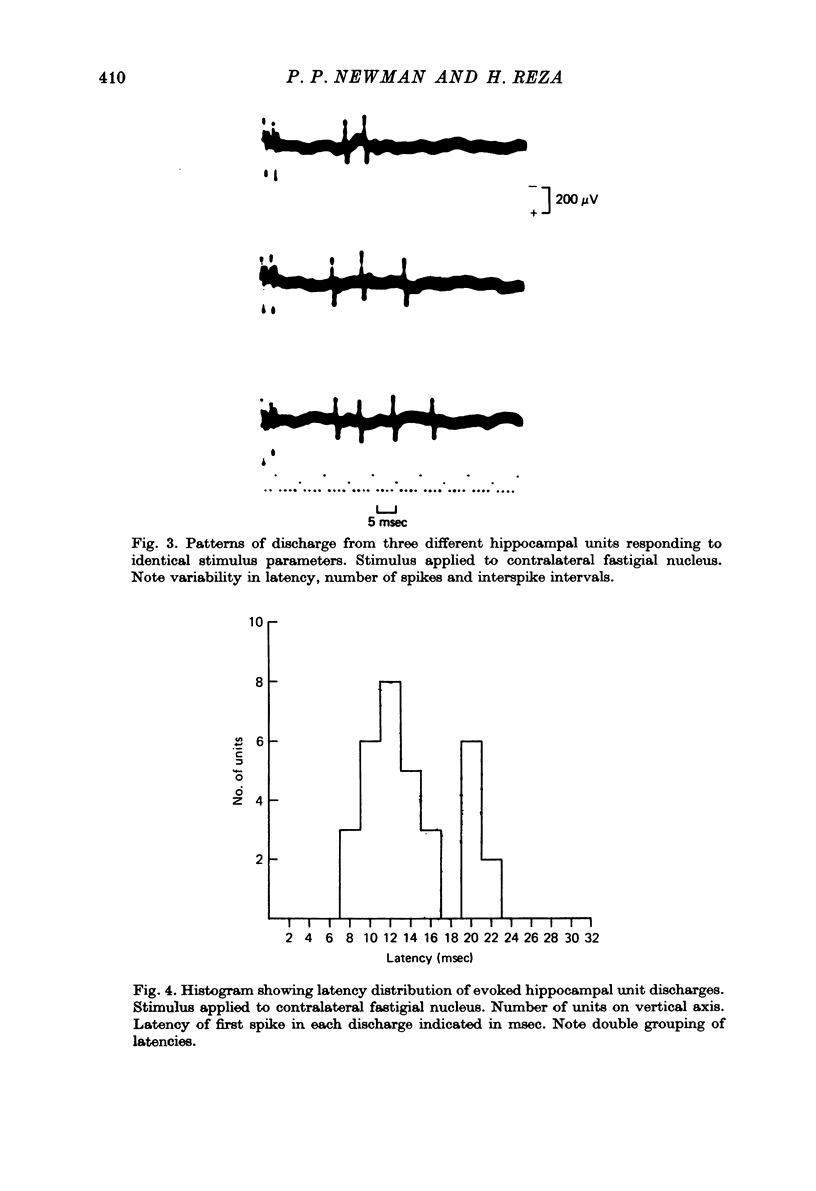
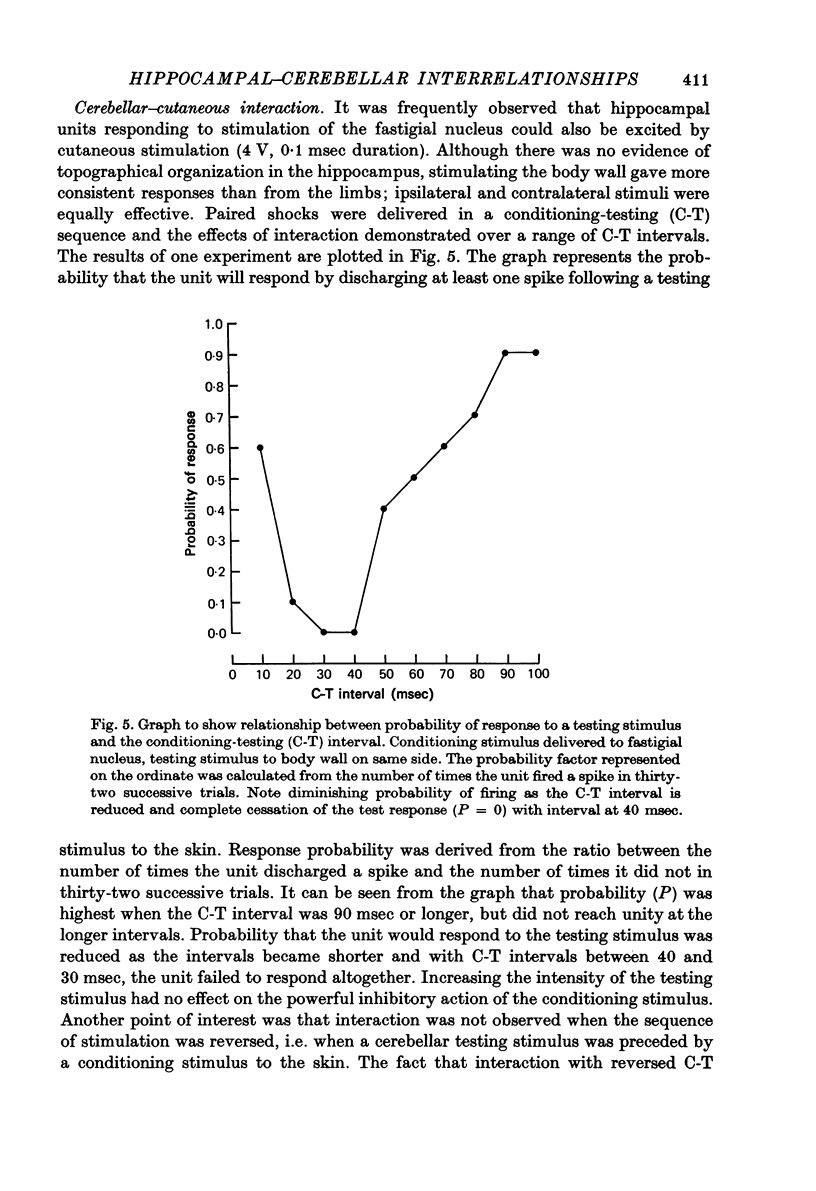
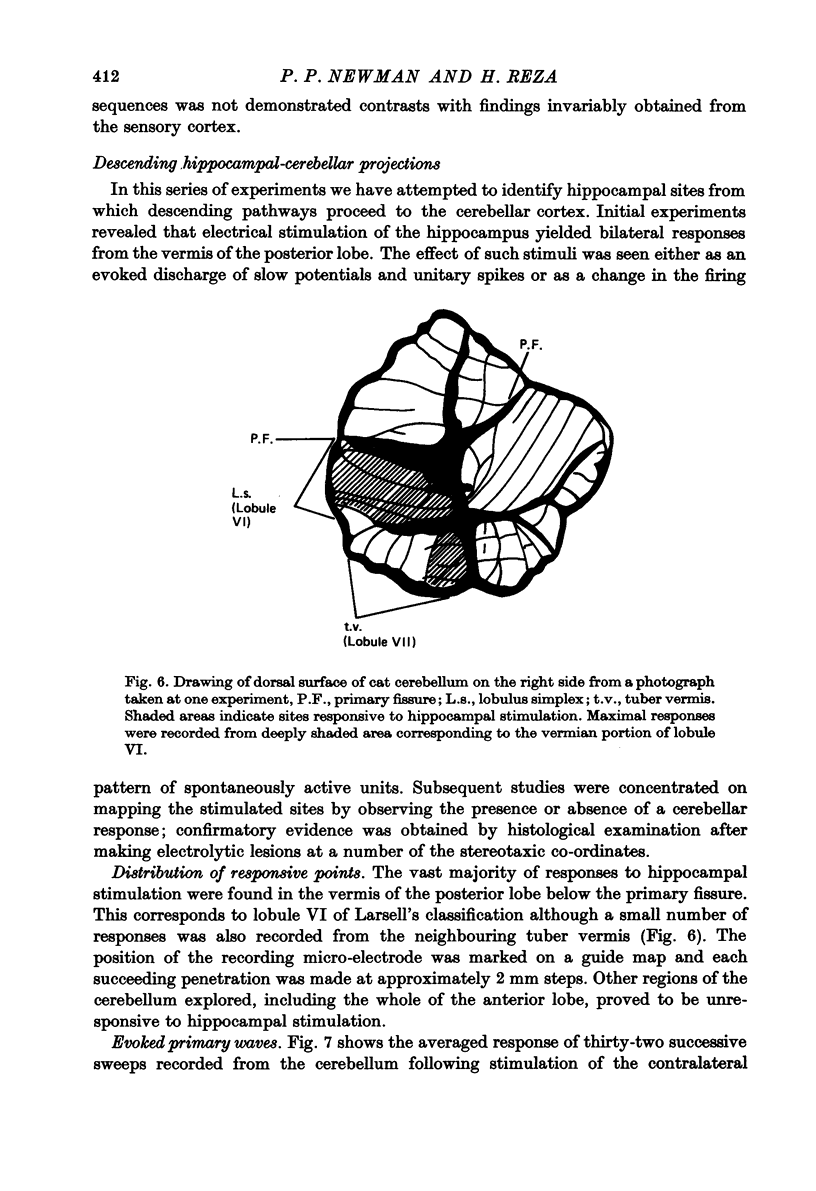
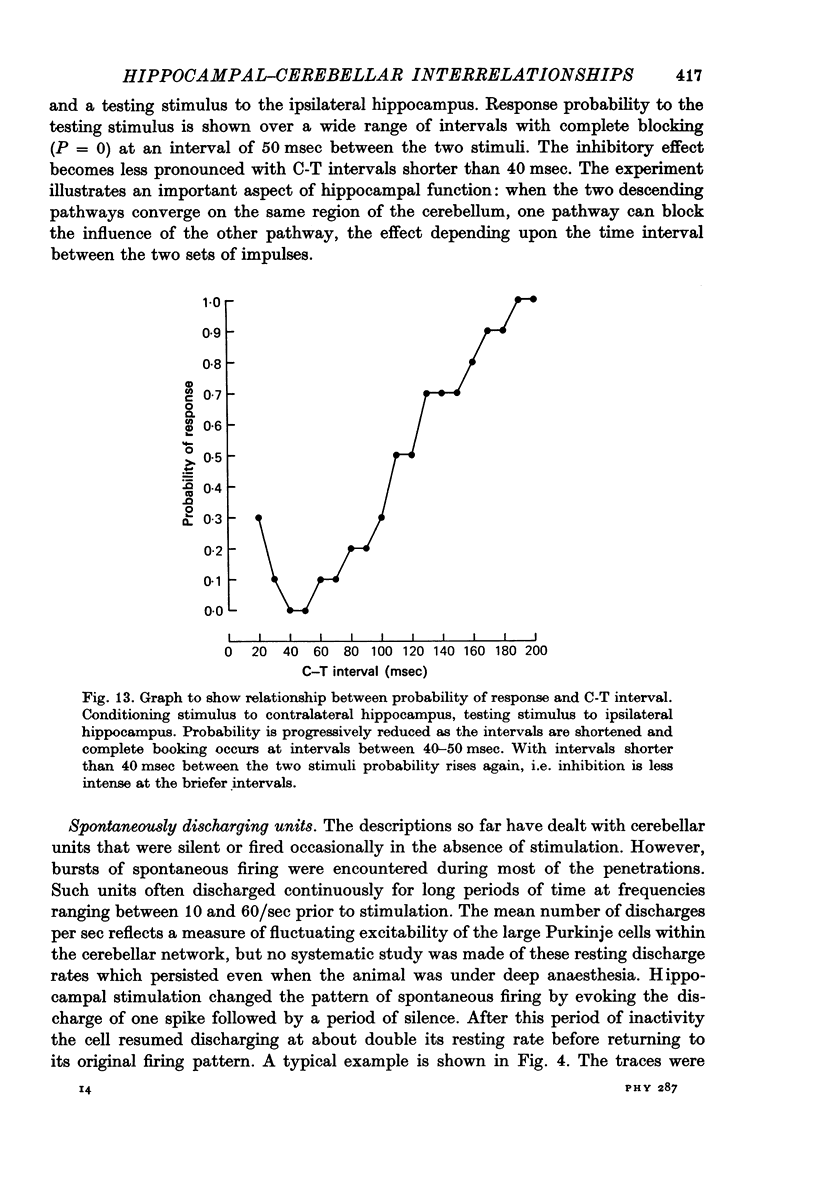
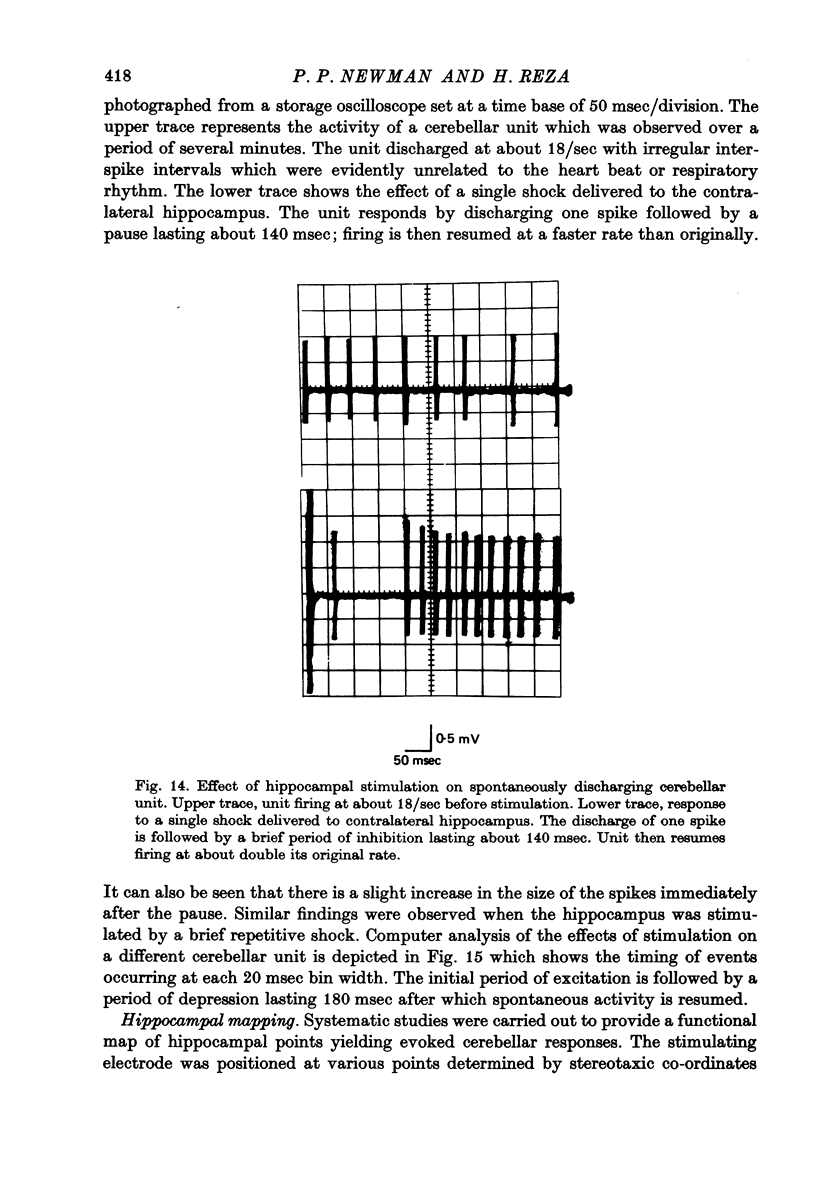
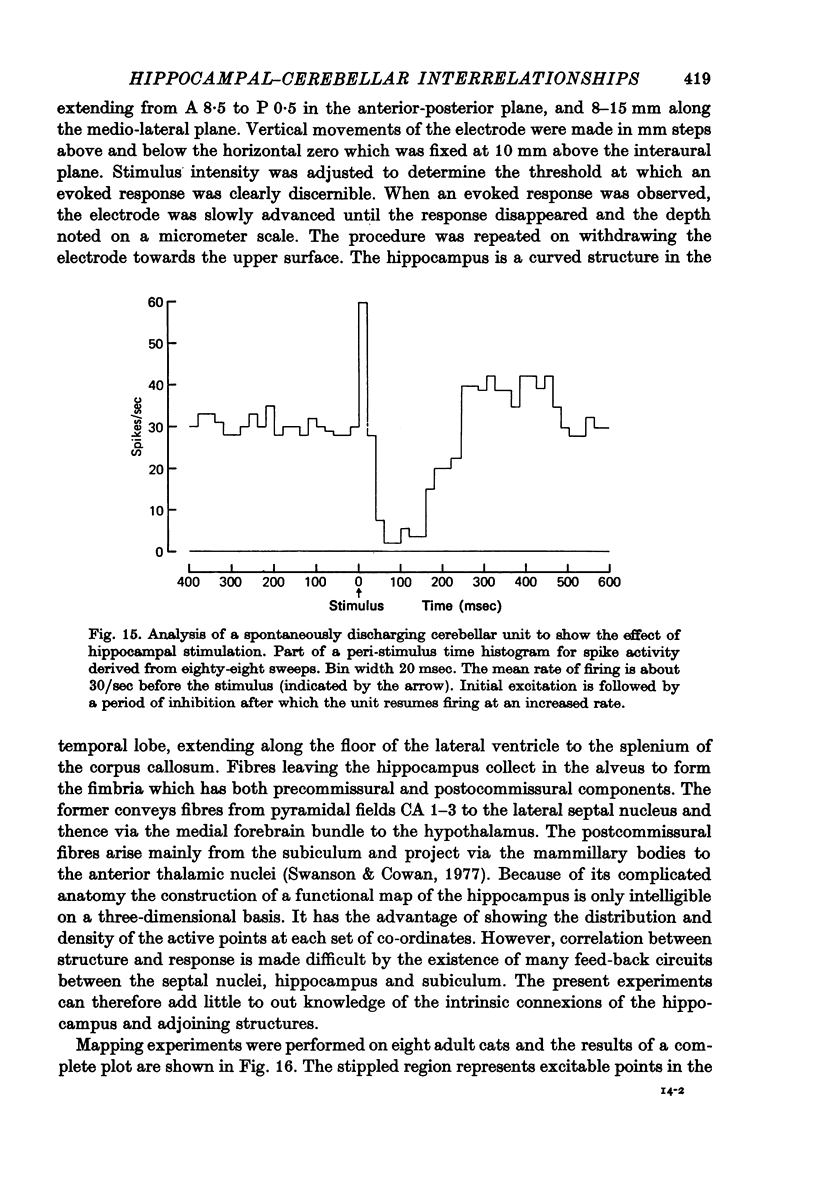
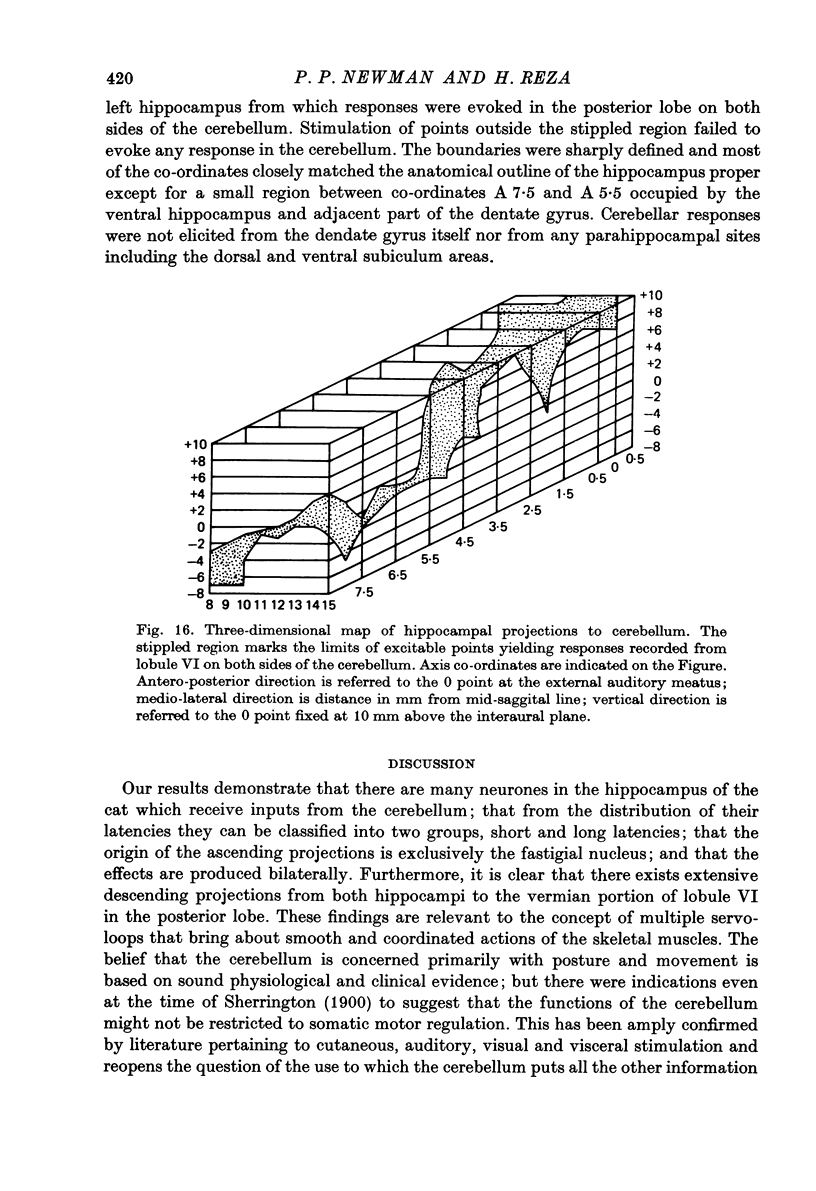
1. Functional interrelationships between the hippocampus and the cerebellum have been investigated in the anaesthetized cat. Plots of the stimulating and recording sites as well as the latency range of the responses indicated the extent of ascending and descending lines of operation between these two structures. 2. Stimulation of the fastigial nucleus evoked the discharge of single hippocampal units on both sides of the brain. Early responses had a mean latency of 12 msec and late responses had a mean latency of 21 msec. Increasing the intensity of the stimulus had little effect on the patterns of discharge. 3. There was no topographical organization within the hippocampus. On the other hand, the activity evoked by a cutaneous stimulus was shown to be greatly depressed by a preceding cerebellar stimulus, particularly at intervals 30--40 msec between the two stimuli. 4. Cerebellar responses evoked by stimulating the hippocampus were found mainly in lobule VI of the posterior lobe. Early and late responses were frequently recorded in the same trace, ipsilateral stimulation yielding the shortest latencies. Increasing the intensity of the stimulus increased the likelihood of there being a response and increased the number of spikes in each discharge. Hippocampal stimulation also had a profound influence on resting cerebellar discharges. 5. Symmetrical points in the two hippocampi were chosen for conditioning and testing sequences. The conditioning stimulus had a long-lasting inhibitory effect on the test response followed by a slow recovery. 6. The location and extent of hippocampal influences on the cerebellum were determined by plotting the presence or absence of a response at each stimulated site. The results indicated the existence of bilateral descending projections containing fast and slow components in conformity with the known conduction properties of mossy fibre and climbing fibre inputs. 7. The physiological significance of interrelationship between the hippocampus and the cerebellum is discussed. It seems that there are many similarities as well as fundamental differences in the cerebellar control of movement under normal circumstances and in conditions of stress.
Full text
PDF



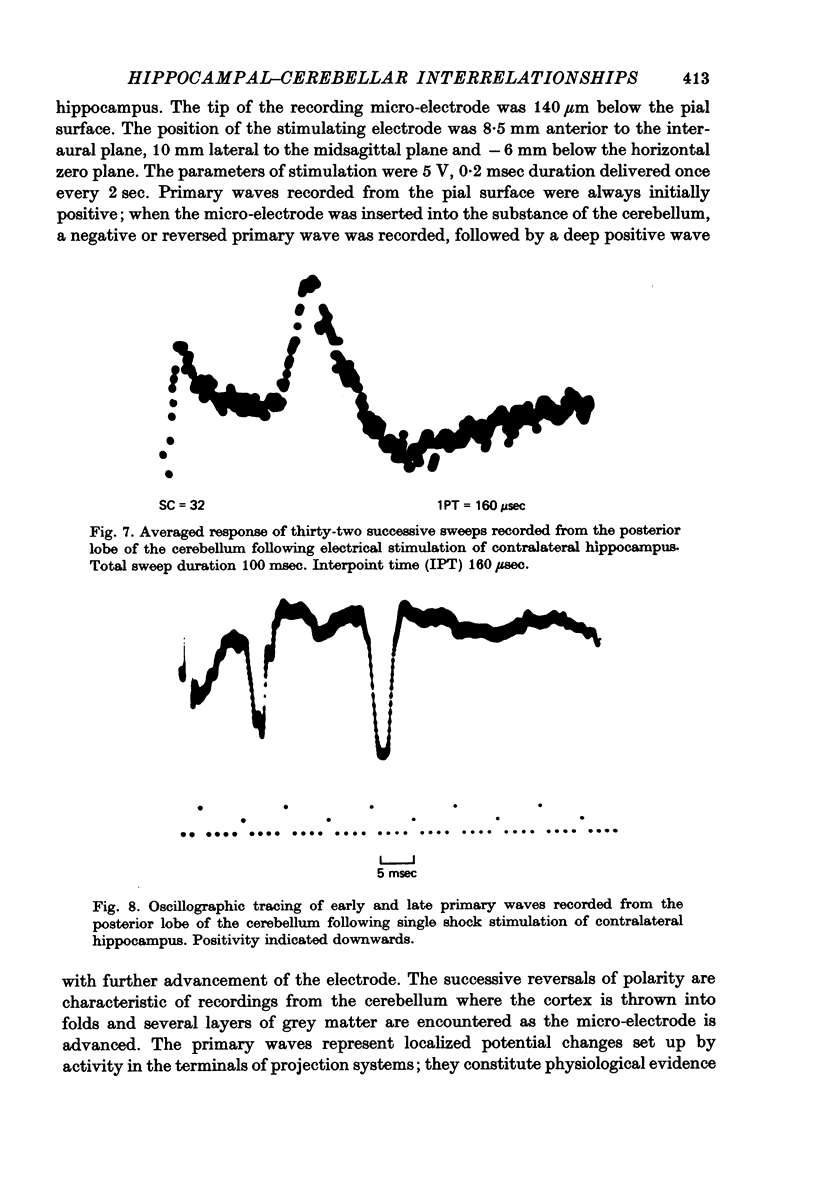
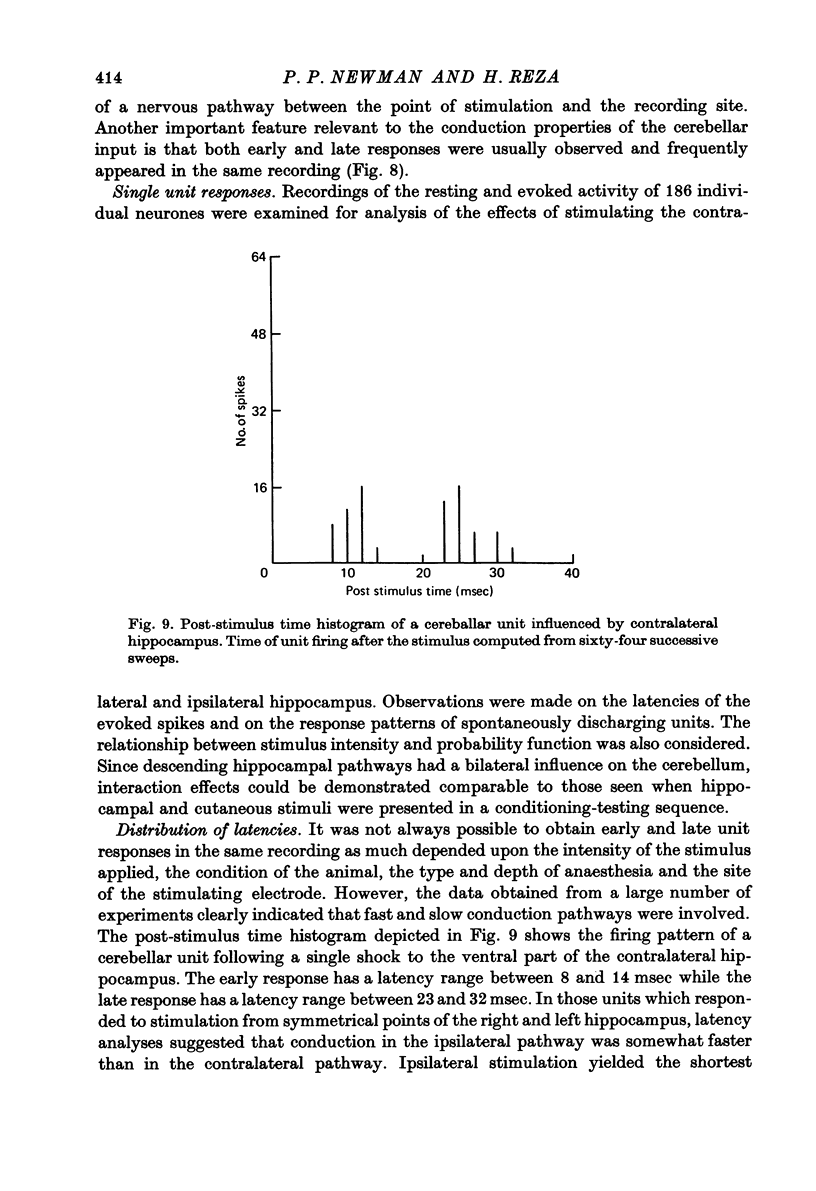
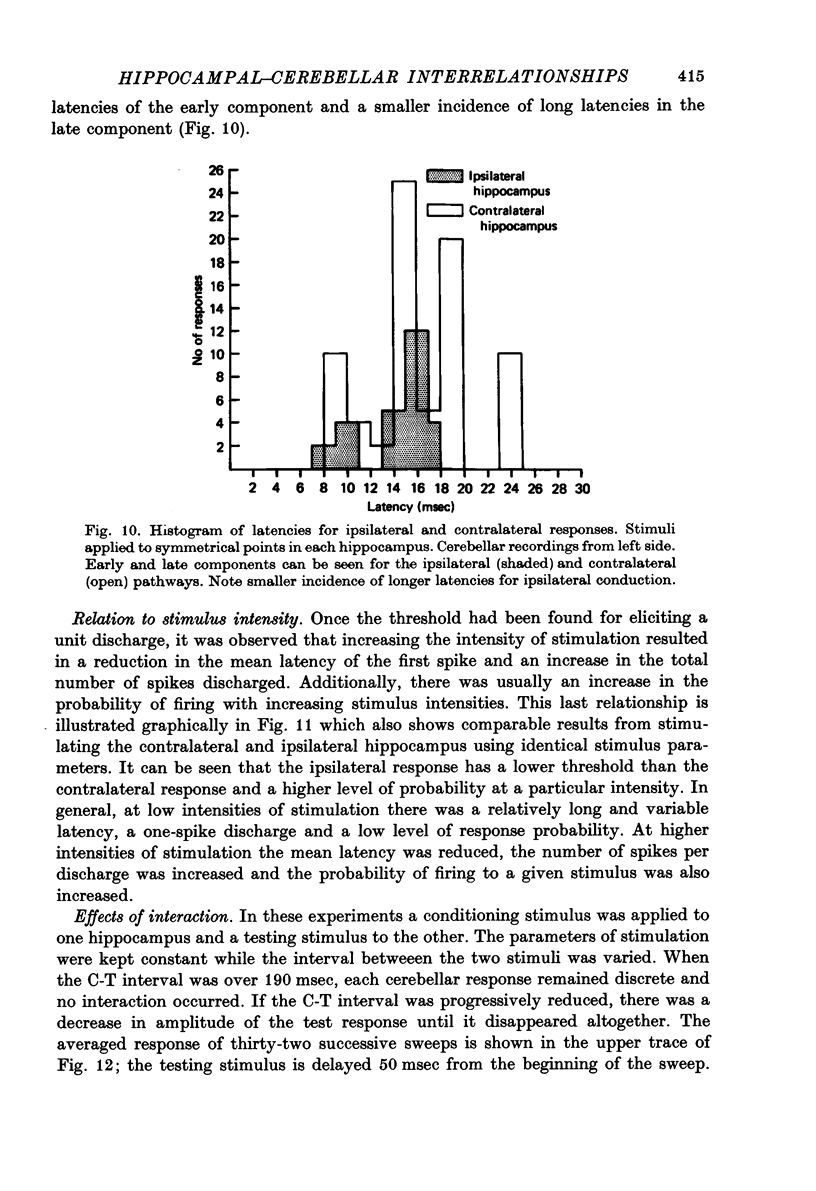
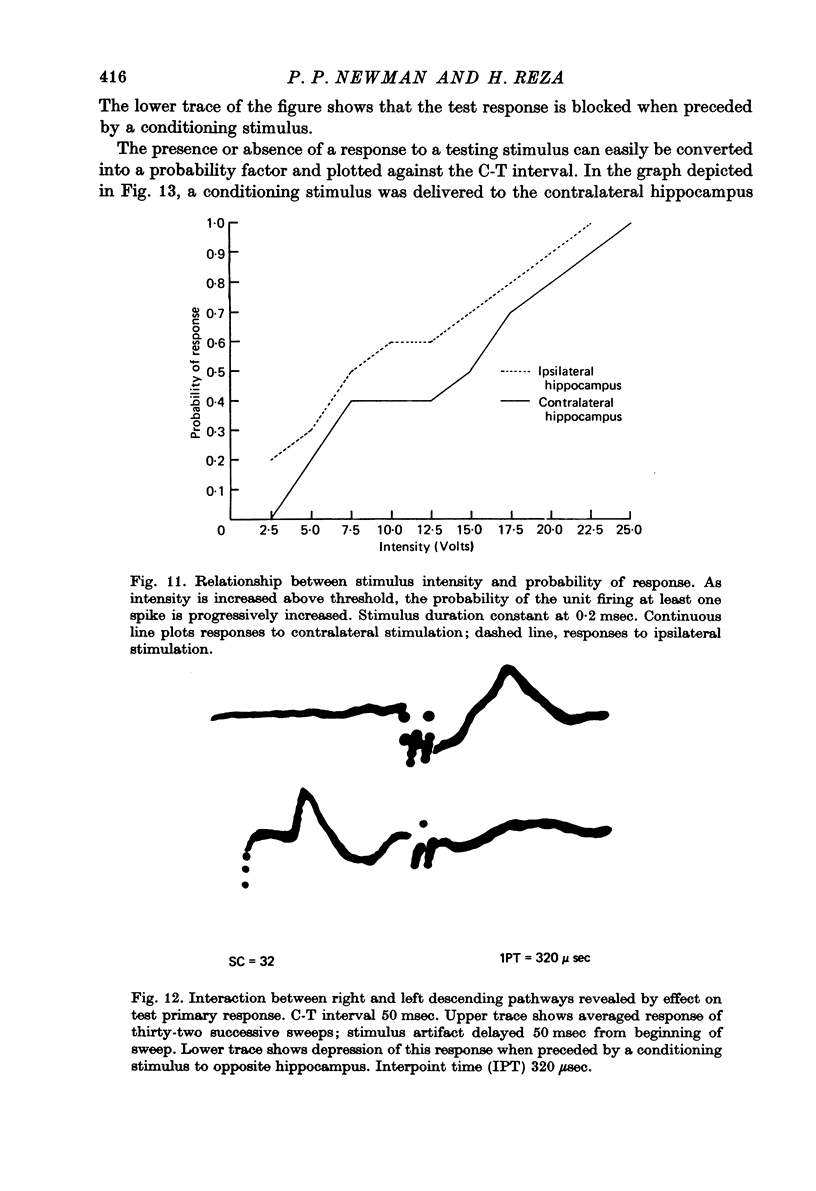






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEY W. R., LIVINGSTON R. B., SEGUNDO J. P. Corticifugal influences on intrinsic brainstem conduction in cat and monkey. J Neurophysiol. 1957 Jan;20(1):1–16. doi: 10.1152/jn.1957.20.1.1. [DOI] [PubMed] [Google Scholar]
- ADEY W. R., MEYER M. Hippocampal and hypothalamic connexions of the temporal lobe in the monkey. Brain. 1952 Sep;75(3):358–384. doi: 10.1093/brain/75.3.358. [DOI] [PubMed] [Google Scholar]
- ANAND B. K., MALHOTRA C. L., SINGH B., DUA S. Cerebellar projections to limbic system. J Neurophysiol. 1959 Jul;22(4):451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Angaut P., Bowsher D. Ascending projections of the medial cerebellar (fastigial) nucleus: an experimental study in the cat. Brain Res. 1970 Nov 11;24(1):49–68. doi: 10.1016/0006-8993(70)90273-8. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., HAMLYN L. H. Action potentials of the pyramidal neurones in the hippocampus of the rabbit. J Physiol. 1955 Sep 28;129(3):608–627. doi: 10.1113/jphysiol.1955.sp005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAGG B. G., HAMLYN L. H. Histologic connections and electrical and autonomic responses evoked by stimulation of the dorsal fornix in the rabbit. Exp Neurol. 1959 Aug;1:187–213. doi: 10.1016/0014-4886(59)90001-9. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Murphy J. T. Convergence of impulses upon single hypothalamic neurons. Brain Res. 1968 Apr;8(1):167–176. doi: 10.1016/0006-8993(68)90178-9. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Mode of operation of the cerebellum in the dynamic loop control of movement. Brain Res. 1972 May 12;40(1):73–80. doi: 10.1016/0006-8993(72)90109-6. [DOI] [PubMed] [Google Scholar]
- FELDMAN S. Neurophysiological mechanisms modifying afferent hypothalamo-hippocampal conduction. Exp Neurol. 1962 Apr;5:269–291. doi: 10.1016/0014-4886(62)90038-9. [DOI] [PubMed] [Google Scholar]
- Fox S. S., Liebeskind J. C., O'Brien J. H., Dingle R. D. Mechanisms for limbic modification of cerebellar and cortical afferent information. Prog Brain Res. 1967;27:254–280. doi: 10.1016/S0079-6123(08)63104-0. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., ADEY W. R. Electrophysiological studies of hippocampal connections and excitability. Electroencephalogr Clin Neurophysiol. 1956 May;8(2):245–263. doi: 10.1016/0013-4694(56)90117-1. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Margnelli M., Mancia M., Grantyn A. Postsynaptic potentials in the mesencephalic and pontomedullar reticular regions underlying descending limbic influences. Brain Res. 1973 Jun 29;56:107–121. doi: 10.1016/0006-8993(73)90329-6. [DOI] [PubMed] [Google Scholar]
- Heath R. G., Harper J. W. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974 Nov;45(2):268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- MORIN F., GREEN J. D. Diffuse after-discharges following stimulation of the fimbria hippocampi. Am J Physiol. 1953 Nov;175(2):251–257. doi: 10.1152/ajplegacy.1953.175.2.251. [DOI] [PubMed] [Google Scholar]
- Meibach R. C., Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977 Jan 1;119(1):1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- NAUTA W. J. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958 Sep;81(3):319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- NIEMER W. T., POWELL E. W., GOODFELLOW E. F. The subcortex and hypothalamic after-discharge in the cat. Electroencephalogr Clin Neurophysiol. 1960 May;12:345–358. doi: 10.1016/0013-4694(60)90009-2. [DOI] [PubMed] [Google Scholar]
- POWELL T. P., COWAN W. M. An experimental study of the efferent connexions of the hippocampus. Brain. 1955;78(1):115–132. doi: 10.1093/brain/78.1.115. [DOI] [PubMed] [Google Scholar]
- RAND R. W. An anatomical and experimental study of the cerebellar nuclei and their efferent pathways in the monkey. J Comp Neurol. 1954 Aug;101(1):167–223. doi: 10.1002/cne.901010107. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977 Mar 1;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Cerebellar output: properties, synthesis and uses. Brain Res. 1972 May 12;40(1):89–102. doi: 10.1016/0006-8993(72)90112-6. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C. H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969 Apr;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- WHITESIDE J. A., SNIDER R. S. Relation of cerebellum to upper brain stem. J Neurophysiol. 1953 Jul;16(4):397–413. doi: 10.1152/jn.1953.16.4.397. [DOI] [PubMed] [Google Scholar]
- YAKOVLEV P. I., LOCKE S., KOSKOFF D. Y., PATTON R. A. Limbic nuclei of thalamus and connections of limbic cortex. Arch Neurol. 1960 Dec;3:620–641. doi: 10.1001/archneur.1960.00450060008002. [DOI] [PubMed] [Google Scholar]


