Abstract
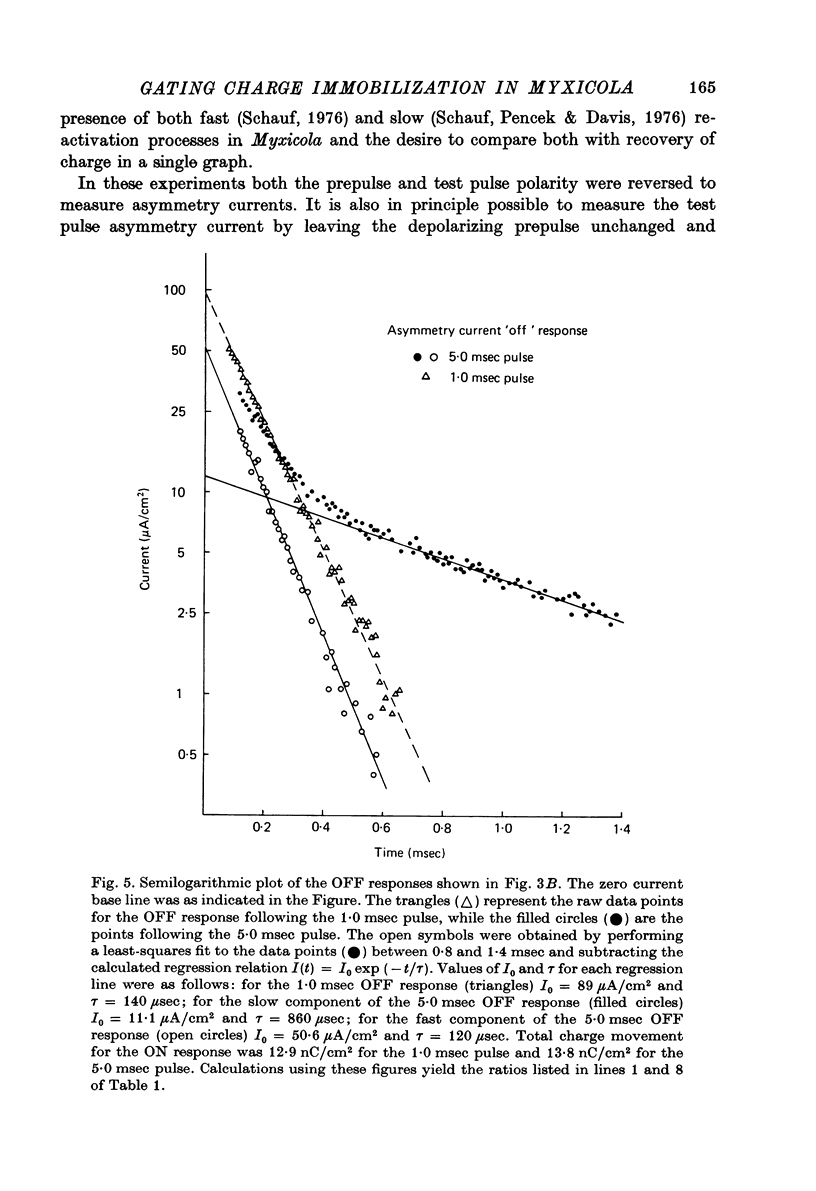
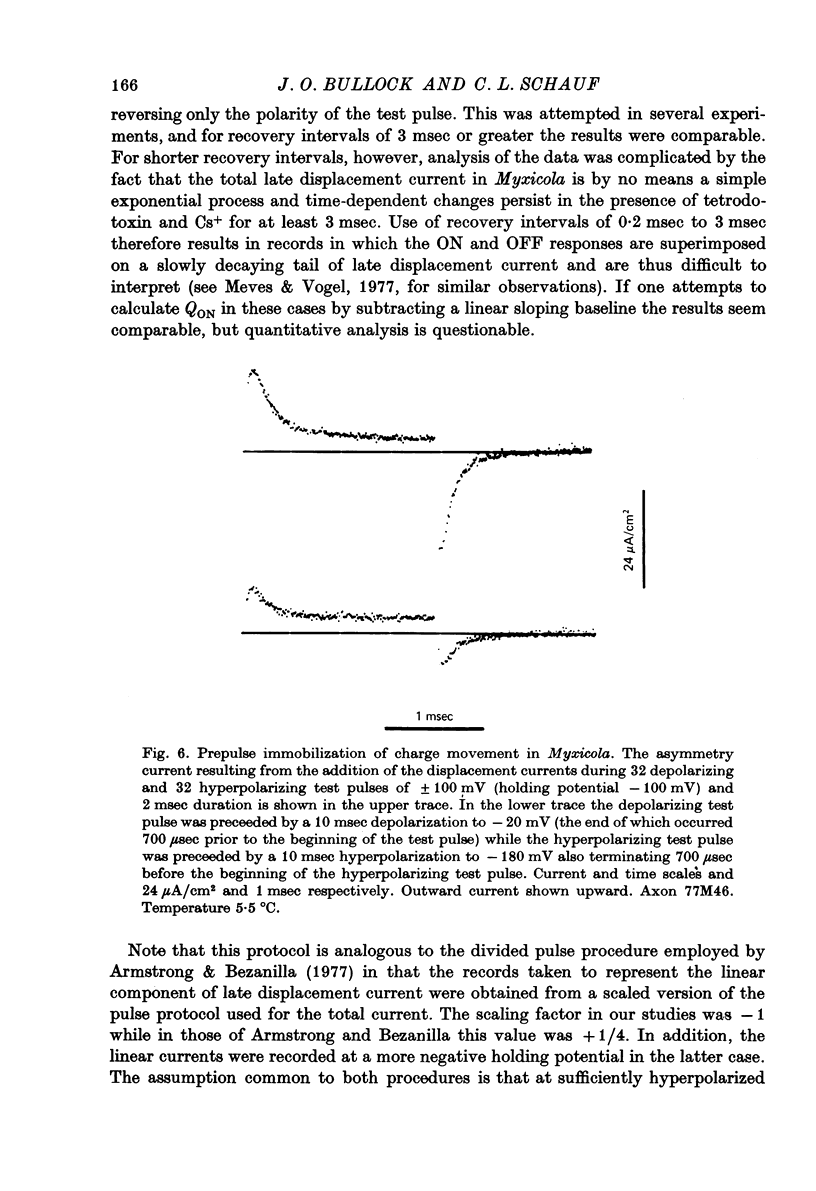
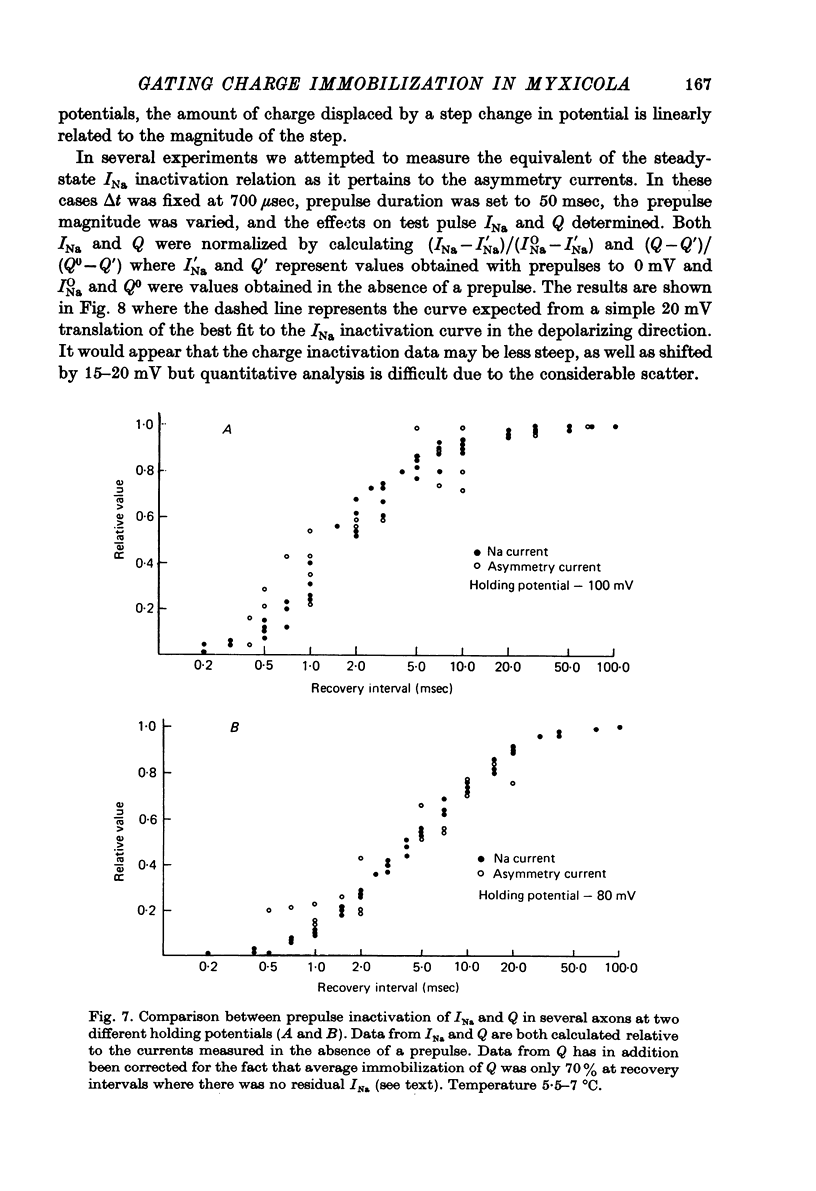
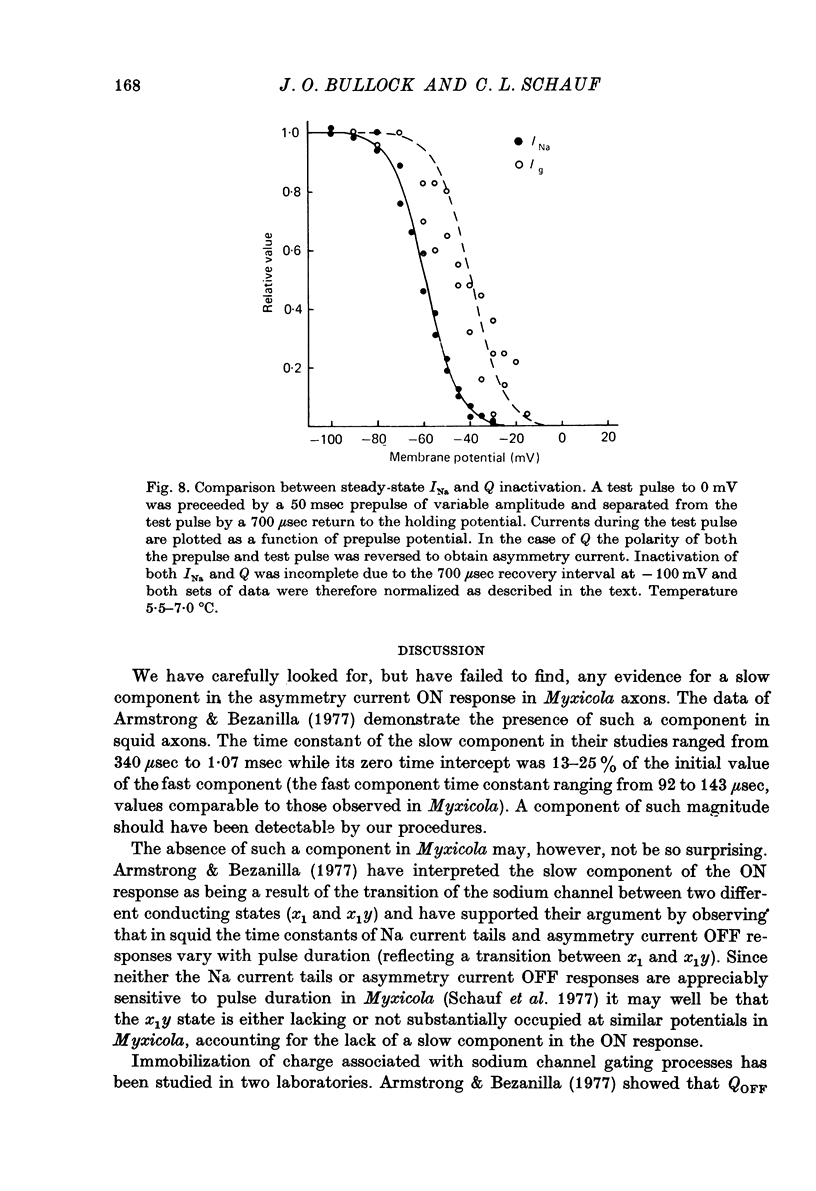
1. Immobilization of gating charge was examined in Myxicola giant axons dialysed with Cs+. 2. With increasing pulse durations the ratio QOFF/QON decreases to as little as 0.25 with a time constant of 3.2 msec determined from composite data. Na inactivation time constants measured in the same axons ranged from 0.8 to 1.5 msec. A slow component of QOFF tends to appear as QOFF/QON decreases. 3. Both QON and QOFF may be decreased by a prepulse with no change in their time course. In this case the time course of the decrease is similar to that observed for INA. 4. The normalized steady-state charge immobilization curve is shifted in the depolarized direction by 15--20 mV from the normalized Na inactivation curve.
Full text
PDF

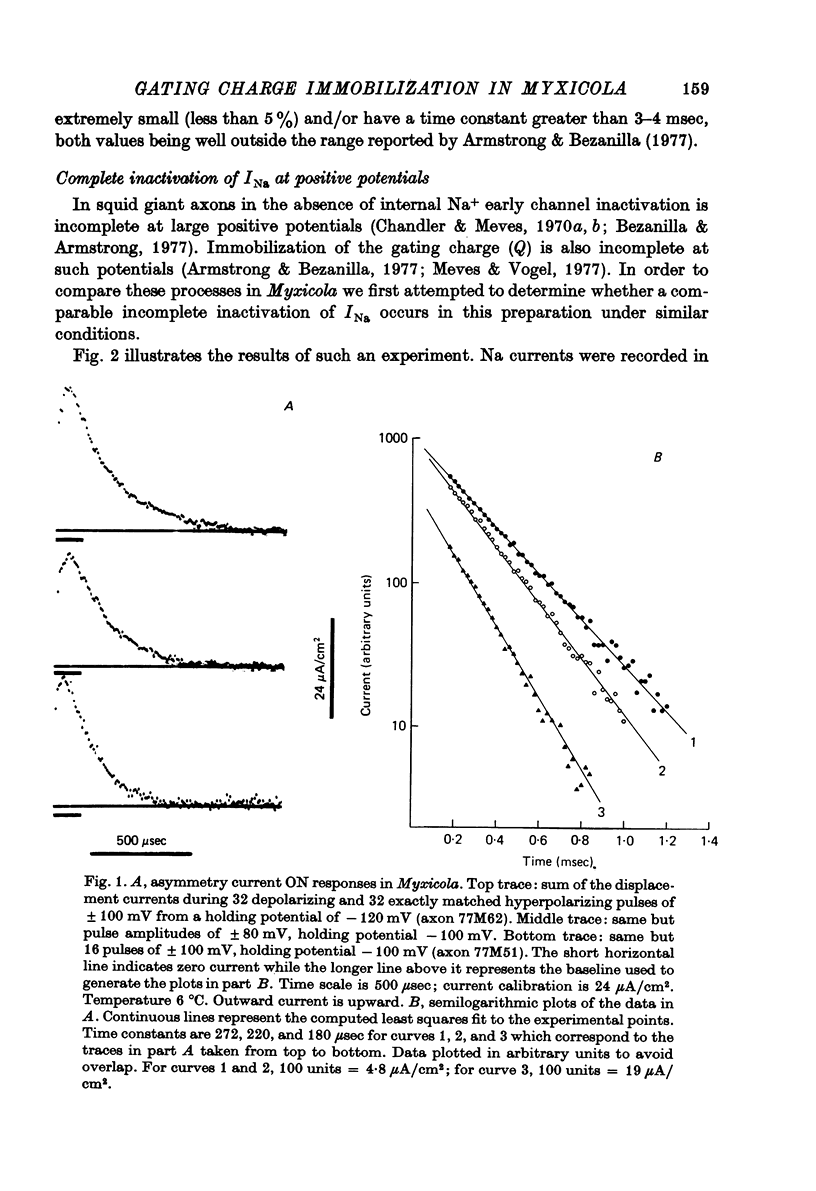
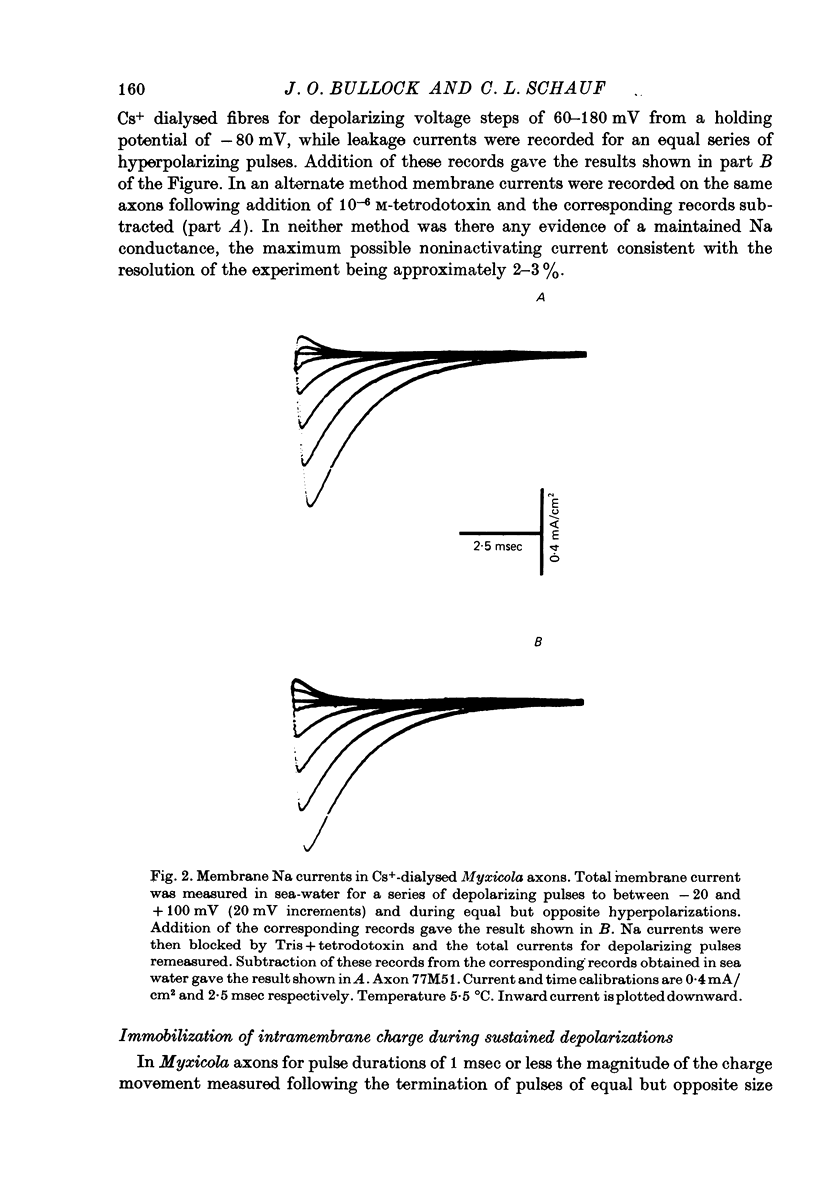
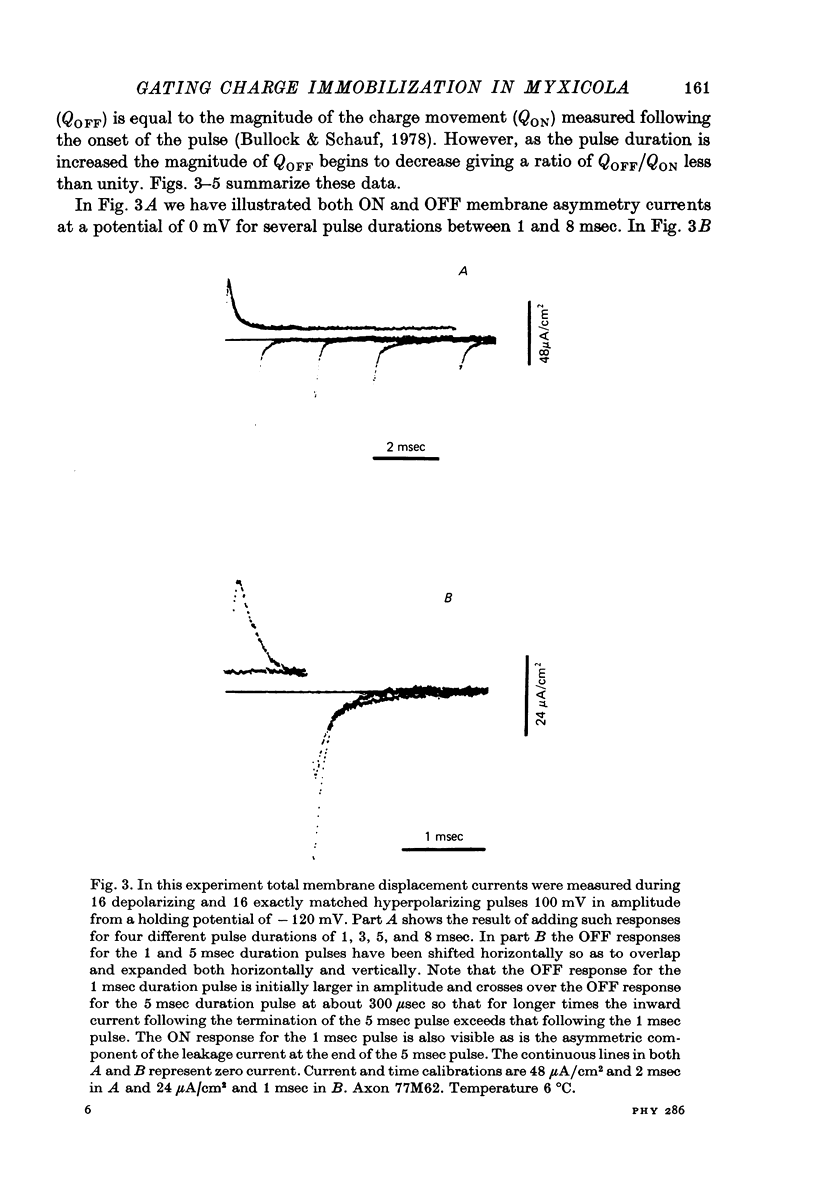
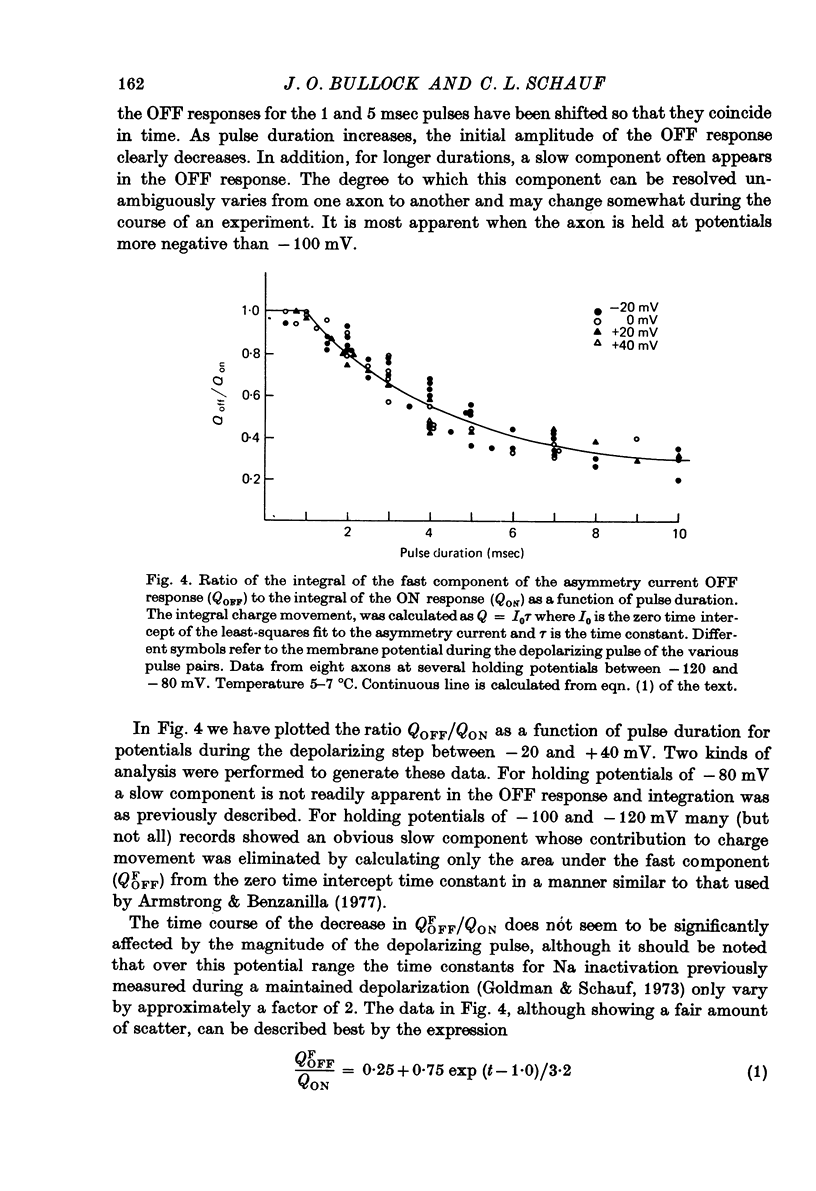





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J. O., Schauf C. L. Combined voltage-clamp and dialysis of Myxicola axons: behaviour of membrane asymmetry currents. J Physiol. 1978 May;278:309–324. doi: 10.1113/jphysiol.1978.sp012306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Inactivation of the asymmetrical displacement current in giant axons of Loligo forbesi. J Physiol. 1977 May;267(2):377–393. doi: 10.1113/jphysiol.1977.sp011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Nonner W., Stämpfli R. Asymmetrical displacement current and its relation with the activation of sodium current in the membrane of frog myelinated nerve. Pflugers Arch. 1976 Jun 22;363(3):193–203. doi: 10.1007/BF00594601. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O., Pencek T. L. Characteristics of sodium tail currents in Myxicola axons. Comparison with membrane asymmetry currents. Biophys J. 1977 Jul;19(1):7–28. doi: 10.1016/S0006-3495(77)85559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Comparison of two-pulse sodium inactivation with reactivation in Myxicola giant axons. Biophys J. 1976 Mar;16(3):245–248. doi: 10.1016/S0006-3495(76)85684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Slow sodium inactivation in Myxicola axons. Evidence for a second inactive state. Biophys J. 1976 Jul;16(7):771–778. doi: 10.1016/S0006-3495(76)85727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


