Abstract
1. The uptake of K by a capillary suspension isolated from rat brain was studied with the radioactive analogue 86Rb.
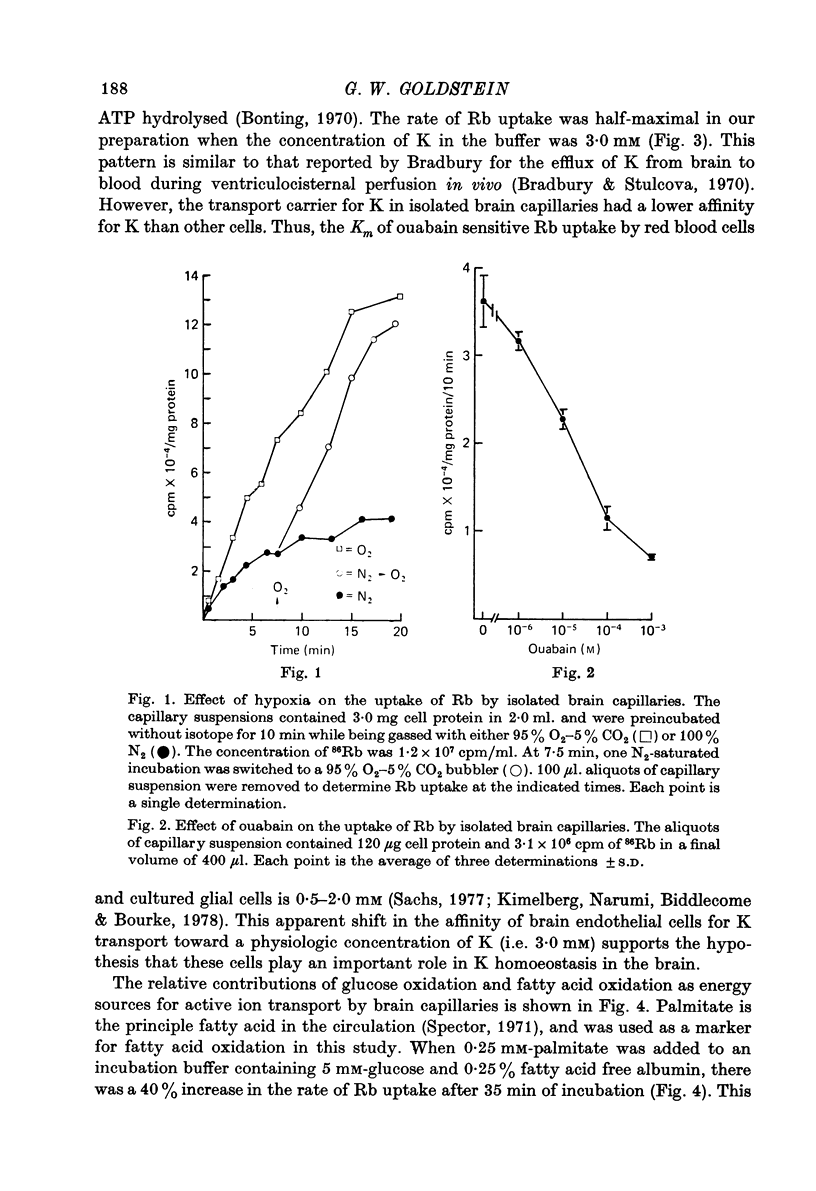
2. Rb uptake was dependent upon the presence of oxygen and could be markedly inhibited with ouabain.
3. The ouabain sensitive Rb uptake was measured at varying external concentrations of K. Uptake of K (as 86Rb) was half-maximal when the K concentration was 3·0 mM. This in vitro affinity of the transport carrier for K is similar to that found in previous in vivo studies of K efflux from brain to blood.
4. I propose that the ouabain sensitive K pump is located on the antiluminal plasma membrane of brain capillary endothelial cells and that this pump contributes to the maintenance of a constant concentration (i.e. 3 mM) of K in brain interstitial fluid.
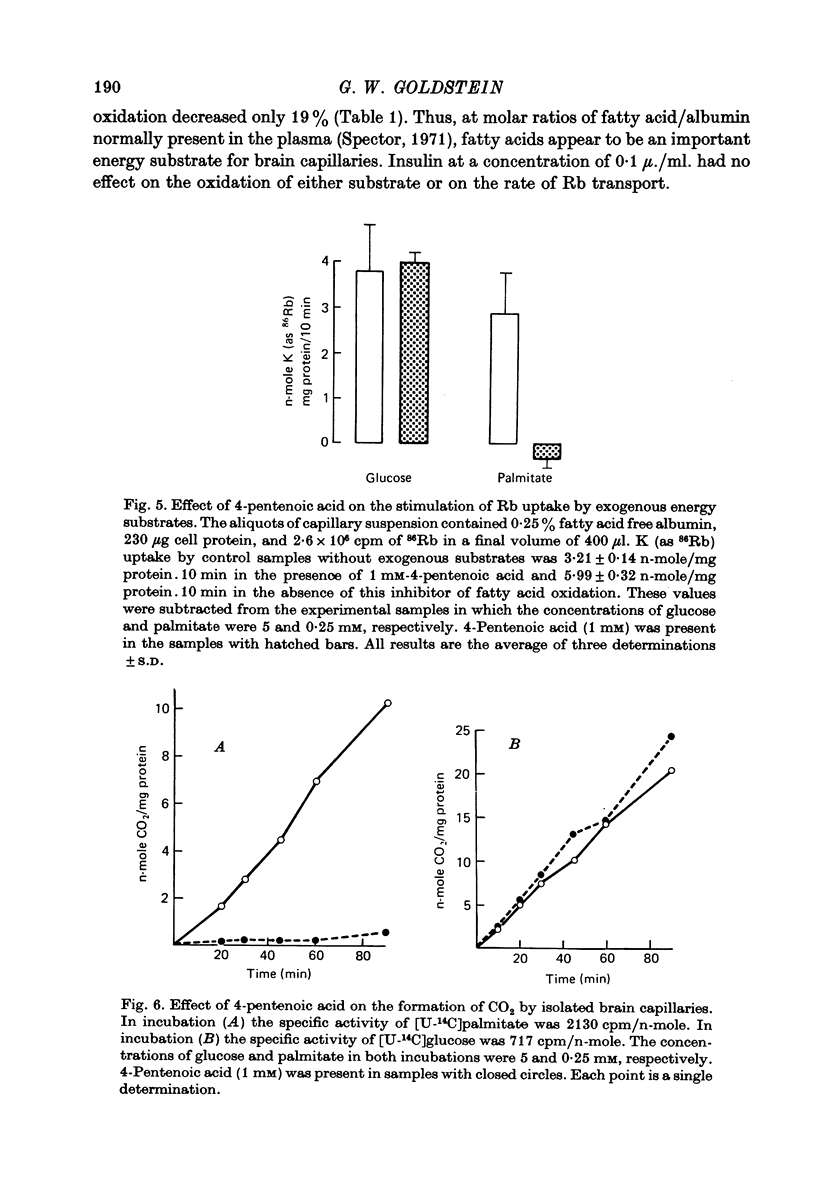
5. Glucose and palmitate were tested as possible energy substrates for the support of active Rb uptake by isolated brain capillaries. The rate of Rb uptake increased 40% when 0·25 mM-palmitate was added to a capillary suspension containing 5 mM-glucose. This stimulation of Rb uptake could be blocked by 1 mM-4-pentenoic acid, an inhibitor of fatty acid oxidation. In contrast, the fraction of Rb uptake supported by glucose was not altered by 4-pentenoic acid.
6. The rates of [U-14C]glucose and [U-14C]palmitate oxidation to CO2 were measured in isolated brain capillaries and compared to their oxidation by brain slices and synaptosomes. Palmitate was the source of 28% of the 14CO2 produced by the capillaries but only 0·5% of the 14CO2 produced by the brain slices and synaptosomes.
7. It is concluded that brain capillaries are similar to renal tubules in their polar distribution of ouabain sensitive K transport carriers, dependence on oxidative metabolism for active ion transport, and use of fatty acids as energy substrates. These features may underlie the vulnerability of brain capillaries in several metabolic diseases that cause brain oedema.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Larsen F. S., Brody T. M. The effect of ouabain on sodium- and potassium-activated adenosine triphosphatase from the hearts of several mammalian species. J Pharmacol Exp Ther. 1969 Nov;170(1):17–26. [PubMed] [Google Scholar]
- Bouldin T. W., Krigman M. R. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 1975 Dec 5;99(2):444–448. doi: 10.1016/0006-8993(75)90053-0. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Segal M. B., Wilson J. Transport of potassium at the blood-brain barrier. J Physiol. 1972 Mar;221(3):617–632. doi: 10.1113/jphysiol.1972.sp009771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Stulcová B. Efflux mechanism contributing to the stability of the potassium concentration in cerebrospinal fluid. J Physiol. 1970 Jun;208(2):415–430. doi: 10.1113/jphysiol.1970.sp009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler R., Corredor C., Brendel K. Hypoglycin and hypoglycin-like compounds. Pharmacol Rev. 1969 Jun;21(2):105–130. [PubMed] [Google Scholar]
- Davson H. Review lecture. The blood-brain barrier. J Physiol. 1976 Feb;255(1):1–28. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo D. C. Reye syndrome: a metabolic response to an acute mitochondrial insult? Neurology. 1978 Feb;28(2):105–108. doi: 10.1212/wnl.28.2.105. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W. Cerebral edema: role of fatty acid metabolism of brain capillaries. N Engl J Med. 1977 Mar 17;296(11):632–633. doi: 10.1056/NEJM197703172961115. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Wolinsky J. S., Csejtey J., Diamond I. Isolation of metabolically active capillaries from rat brain. J Neurochem. 1975 Nov;25(5):715–717. doi: 10.1111/j.1471-4159.1975.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Lund-Andersen H., Crone C. K+-permeability of the blood-brain barrier, investigated by aid of a K+-sensitive microelectrode. Acta Physiol Scand. 1977 Dec;101(4):438–445. doi: 10.1111/j.1748-1716.1977.tb06027.x. [DOI] [PubMed] [Google Scholar]
- Katzman R. Maintenance of a constant brain extracellular potassium. Fed Proc. 1976 May 1;35(6):1244–1247. [PubMed] [Google Scholar]
- Kinne R., Murer H., Kinne-Saffran E., Thees M., Sachs G. Sugar transport by renal plasma membrane vesicles. Characterization of the systems in the brush-border microvilli and basal-lateral plasma membranes. J Membr Biol. 1975;21(3-4):375–395. doi: 10.1007/BF01941077. [DOI] [PubMed] [Google Scholar]
- Kleinman J. G., Mandelbaum J., Levin M. L. Renal functional effects of 4-pentenoic acid, an inhibitor of fatty acid oxidation. Am J Physiol. 1973 Jan;224(1):95–101. doi: 10.1152/ajplegacy.1973.224.1.95. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILSTEIN S. W., DRISCOLL L. H. Oxidation of albumin-bound palmitate-1-C14 by adipose and hepatic tissues of the rat. J Biol Chem. 1959 Jan;234(1):19–21. [PubMed] [Google Scholar]
- Milhorat T. H., Hammock M. K., Fenstermacher J. D., Levin V. A. Cerebrospinal fluid production by the choroid plexus and brain. Science. 1971 Jul 23;173(3994):330–332. doi: 10.1126/science.173.3994.330. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Cornford M. E., Brown W. J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977 May;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Kinetic evaluation of the Na-K pump reaction mechanism. J Physiol. 1977 Dec;273(2):489–514. doi: 10.1113/jphysiol.1977.sp012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Kean E. A., Johnson B. Jamaican vomiting sickness. Biochemical investigation of two cases. N Engl J Med. 1976 Aug 26;295(9):461–467. doi: 10.1056/NEJM197608262950901. [DOI] [PubMed] [Google Scholar]
- Vaughan G. L., Cook J. S. Regeneration of cation-transport capacity in HeLa cell membranes after specific blockade by ouabain. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2627–2631. doi: 10.1073/pnas.69.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Bradley R. F. Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med. 1967 Mar 23;276(12):665–669. doi: 10.1056/NEJM196703232761204. [DOI] [PubMed] [Google Scholar]


