Abstract
Neutrophil (PMN) functions can be primed for greatly increased oxidative radical release by exposure to certain agents such as lipopolysaccharide (LPS). Although a variety of signaling pathways involving both tyrosine kinases and mitogen-activated protein (MAP) kinases may be operative, the mechanisms of PMN priming are still not understood. We found that PMN priming was not achieved by treatment of cells with a very low concentration (5 ng/ml) of LPS unless additional “helper” factors were present in plasma (5%). Under these conditions, LPS induced tyrosine phosphorylation of a 38-kDa protein, which was coincident with the MAP kinase p38 action in this situation. LPS-mediated activation of p38 in human PMNs was dependent on the presence of LPS binding protein from plasma and CD14 on the surfaces of the cells. Phosphorylation of p38 was highly correlated with LPS priming of a formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated PMN respiratory burst. Treatment of PMN with the p38-specific inhibitor SB203580 significantly attenuated the respiratory burst in cells primed by LPS and stimulated by fMLP. These results suggest that the LPS signaling pathway leading to p38 activation may be an important mechanism in regulation of PMN priming. The mediator(s) linking CD14 to p38 involves proteins that are functionally sensitive to genistein but insensitive to tyrphostin AG126 and to Src- and Syk-family kinase, protein kinase C, and phosphatidylinositol 3-kinase inhibitors. Elucidating this pathway will provide insight into possible regulation of PMN priming by LPS.
Infection by gram-negative bacteria remains a global problem, especially for the very young, the elderly, or the immune compromised, in whom septicemia and shock leading to multiple organ failure can occur (27). One important feature of septicemia is the presence in blood of lipopolysaccharide (LPS), a membrane glycolipid from the cell walls of gram-negative bacteria. LPS can be detected in the blood of septicemic patients at nanogram-per-milliliter levels (10, 42). LPS accounts for many symptoms of septic shock, including vasodilation, myocardial dysfunction, and disseminated intravascular coagulation.
In addition to its direct cytotoxic effects on endothelium, LPS also affects a variety of cellular functions in blood cells (1). One example is priming of polymorphonuclear neutrophils (PMN). When preexposed to LPS, PMN are primed, i.e., poised for a dramatically increased level of oxidative radical production elicited by a very weak secondary stimulus, such as the chemotactic factor formyl-methionyl-leucyl-phenylalanine (fMLP) (34). Although the excessive production of reactive oxygen radicals by PMN is necessary for effective killing of invading organisms, it is also associated with tissue damage during inflammation (36). In order to devise more-effective treatment strategies, it is important to understand the microbe-host cell interaction, including the mechanism involved in the LPS priming of PMN function.
Our previous studies have demonstrated that LPS priming of human PMN needs plasma to operate effectively (3, 4), indicating the involvement of some plasma factor(s) in the LPS-PMN interaction. LPS binding protein (LBP), an acute-phase plasma protein, binds to LPS, leading to efficient LPS interaction with the cell surface receptor CD14 (45, 46). This complex of LPS-LBP and CD14 then interacts with Toll-like protein 4 (TLR4), by which transmembrane signals are generated to affect cellular functions (16, 40). Recent studies have linked LPS stimulation to mitogen-activated protein (MAP) kinase signaling pathways including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (3, 9, 23-25). The biological function of this family of protein kinases in human PMN, although not definitively determined, has been implicated in the regulation of chemotactic migration, cytoskeletal rearangement, respiratory burst, degranulation, cytokine gene expression, and apoptosis (6).
Human PMN express the α isoform of p38, whose activation is mediated by dual phosphorylation by upstream kinases such as MKK3/MKK6 (12, 24). Once activated, p38 phosphorylates a number of substrates, including activating transcription factor-2 (ATF-2), MAP kinase-activated protein (MAPKAP) kinase 2, MAPKAP kinase 3, c-Jun, ELK-1, and myelin basic protein (12, 24); most of these are transcription factors required for the expression of various genes involved in inflammatory processes. Roles for p38 in PMN priming by LPS, tumor necrosis factor (TNF), or interleukin-8 (IL-8) (49), activation of NADPH oxidase, adhesion, and migration (7, 37) have been proposed, but the mechanism by which the LPS signal is processed to this kinase has yet to be elucidated.
In this study, we simultaneously assessed the activation of p38 and priming of fMLP-induced superoxide anion (O2−) production in human PMN by LPS. The results demonstrated that the plasma factor LBP and cell surface receptor CD14 were necessary for LPS activation of p38, which was tightly associated with LPS priming of the PMN respiratory burst.
MATERIALS AND METHODS
Materials.
Endotoxin-free reagents and plastics were used in all experiments. Acid citrate dextrose was purchased from Biosource (Dartmouth, Canada). Dextran (6%) and human infusion grade water (used for cell isolation and medium preparation) were purchased from Abbott Laboratories Inc. (Toronto, Canada). Ficoll-Paque Plus was purchased from Amersham Pharmacia Biotech, Inc. (Quebec, Canada). Hanks' buffered salt solution, 7.5% sodium bicarbonate, and 1 M HEPES buffer solution were purchased from Gibco Laboratories (Burlington, Canada). SB203580, genistein, tyrphostin AG126, PP2, wortmannin, piceatannol, and calphostin C were obtained from Calbiochem (Hornby, Canada). Escherichia coli LPS (serotype O111:B4) was obtained from List Biological Laboratories Inc. (Campbell, Calif.). Purified natural human LBP and a mouse monoclonal antibody (MAb) to human LBP (immunoglobulin G1 [IgG1]) were obtained from Cedar Lane Laboratories (Hornby, Canada). A rabbit anti-phosphorylated p38 antibody (Ab) was obtained from New England Biolabs. Rabbit anti-p53/56lyn, anti-p59hck, and anti-p38 protein Abs and mouse anti-phosphotyrosine MAb PY99 were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). A rabbit anti-p58fgr Ab was kindly provided by G. Berton (University of Verona, Verona, Italy) (48). Nitrocellulose membranes, ECL Western blot detection reagents, and [γ-32P]ATP were from Amersham Pharmacia Biotech, Inc. (Québec, Canada). The protein assay kit (Bradford method) and other reagents for electrophoresis were from Bio-Rad Laboratories (Canada) Ltd. (Mississauga, Canada). Horseradish peroxidase-conjugated goat anti-mouse IgG and anti-rabbit IgG Abs and all other reagents were obtained from Sigma-Aldrich Canada Ltd. (Oakville, Canada).
Human plasma preparation.
Autologous human plasma was obtained from the citrated whole blood of the same donors whose blood was used for isolation of PMN by centrifugation at 500 × g for 20 min to remove the blood cells. In some experiments, plasma was fractionated (0.5 ml/fraction) by passage through a 30-ml Sephacryl S-200 column which was equilibrated and eluted with H-medium (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.8 mM CaCl2, 5 mM glucose, and 10 mM HEPES [pH 7.4]). As indicated in Results, plasma was preincubated in some cases with 20 μg of the anti-LBP MAb (IgG1)/ml or with a control mouse IgG1 for 1 h at room temperature before addition to cells.
PMN isolation, incubation, and lysis.
Human PMN were isolated from citrated whole blood of healthy volunteers by venipuncture using dextran sedimentation, centrifugation over Ficoll-Paque, and lysis of contaminated erythrocytes with hypotonic saline, as previously described (3). Isolated PMN were suspended at a density of 107/ml in H-medium supplemented with 5% normal autologous plasma, antibody-pretreated plasma, or 20% fractionated plasma. In some experiments, PMN were pretreated with an anti-CD29 (β1 integrin), anti-CD18 (β2 integrin), or anti-CD14 MAb (at 5 μg/ml) or normal mouse IgG for 45 min at 4°C, or with 5 μM SB203580 for 10 min at room temperature before stimulation. Alternatively, some PMN were pretreated with either 50 μM genistein, 5 μM PP2, 5 μM piceatannol, 2 μM calphostin C, 100 nM wortmannin, or 50 μM tyrphostin AG126. Cells were then incubated in 1.5-ml polypropylene microcentrifuge tubes (0.6 ml/tube) with or without addition of 5 ng of LPS/ml for 20 min at 37°C.
After incubation, PMN were collected by centrifugation at 9,300 × g for 10 s in a microcentrifuge and washed once with ice-cold phosphate-buffered saline containing 1 mM diisopropylfluorophosphate (DFP). Cell pellets were lysed with radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM EGTA, 200 μM sodium orthovanadate, 10 μM phenylarsine oxide, 1 mM sodium fluoride, 5 μg of leupeptin/ml, 25 μg of aprotinin/ml, 5 μg of pepstatin A/ml, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) for 30 min on ice, followed by centrifugation at 12,000 × g for 10 min at 4°C to remove insoluble nuclei and granules. Protein concentrations in the lysates were determined by a Bradford protein assay (Bio-Rad Life Science Products) according to the manufacturer's instructions, and lysates were stored at −70°C before use.
SDS-PAGE and Western blotting.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting, as well as membrane stripping and reprobing, were carried out exactly as described previously (3). The antibodies used in the present study were a mouse anti-phosphotyrosine MAb (PY99), a rabbit anti-phospho-p38 Ab (p-p38), and a rabbit anti-p38 protein Ab. Bound Abs were detected with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG followed by treatment with ECL Western blotting reagents and exposure to Kodak X-ray films.
Protein tyrosine kinase assay.
Protein tyrosine kinase activity was detected by an in vitro immunocomplex kinase assay as described previously (48). Briefly, protein tyrosine kinases (Fgr, Hck, and Lyn) were precipitated from the lysates (100 μg in 400 μl) by using specific Abs immobilized on protein A-agarose. Kinase activities were assayed on the washed immunoprecipitates in the presence of [γ-32P]ATP and were analyzed by SDS-PAGE followed by autoradiography.
Superoxide assay.
PMN were pretreated with antibodies or inhibitors as described in Results and were resuspended in H-medium containing 80 μM cytochrome c either alone or supplemented with 5% normal plasma or plasma treated with antibodies (anti-LBP or control IgG), or with 5 ng of purified human LBP/ml. Cells were dispensed into polypropylene microcentrifuge tubes (1.0 ml/tube) and either left unprimed or primed with LPS (5 ng/ml) for 20 min at 37°C, followed by stimulation (or not) with fMLP (10−6 M) for another 5 min. Superoxide (O2−) production was measured by the superoxide dismutase-inhibitable reduction of cytochrome c as described previously (47).
Statistical analysis.
Results were analyzed by one-way analysis of variance to determine the differences between the individual treatments. Statistical significance was defined as a P value of <0.05.
RESULTS
LPS priming of PMN is associated with marked tyrosine phosphorylation of a 38-kDa cellular protein and the MAP kinase p38 in a plasma-dependent manner.
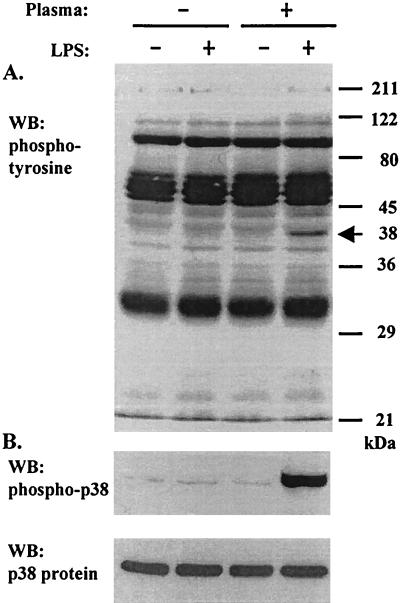
It has long been noted that PMN function, especially the respiratory burst, can be dramatically enhanced by priming with variety of agents, including LPS (34). However, the mechanisms involved in the priming processes are still not fully understood. We previously reported that LPS, when used at a low concentration (5 ng/ml), failed to prime PMN oxidative burst activity in the absence of plasma (4). As shown in Fig. 1A, without the addition of plasma in the incubation medium, treatment of PMN with LPS (5 ng/ml for 20 min at 37°C) alone caused minimal change in total cellular protein tyrosine phosphorylation. In contrast, LPS induced marked tyrosine phosphorylation of a protein(s) migrating at a molecular mass of 38 kDa in cells incubated in a medium containing plasma (Fig. 1A). Since this protein(s) migrated at a molecular size similar to that of the MAP kinase p38, we examined the activation of p38 under our experimental conditions using an antibody specific for activated (i.e., phosphorylated) p38 by Western blotting. As demonstrated in the middle panel of Figure 1, a highly phosphorylated p38 was induced by LPS in PMN incubated in the presence of plasma only, indicating an association and possible role of p38 in LPS priming of PMN function and the involvement of certain plasma factors in the process.
FIG. 1.
LPS increases tyrosine phosphorylation of a cellular 38-kDa protein (indicated by the arrow in panel A) that is coincident with the activation of p38 in human PMN. Purified PMN were resuspended in H-medium alone or supplemented with 5% plasma at a density of 10 × 106/ml and were incubated at 37°C for 20 min in the absence or presence of 5 ng of LPS/ml. Cells were washed once with ice-cold phosphate-buffered saline-2.5 mM DFP, followed by lysis with RIPA buffer. Cellular proteins were analyzed by SDS-PAGE and Western blotting (WB) for tyrosine phosphorylation (A) and p38 activation (B). Equal loading was confirmed by stripping the membrane and reprobing for p38 protein (bottom panel).
LBP is the plasma factor supporting LPS activation of p38 and priming of the respiratory burst in human PMN.
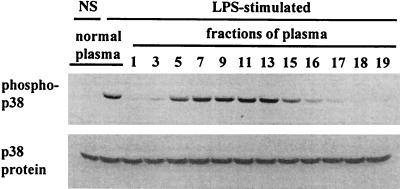
In order to identify the plasma factor(s) that promoted PMN priming by LPS, we fractionated human plasma using a Sephacryl S-200 column. Fractionation (0.5-ml aliquots) began when proteins were first eluted and was ended when no protein was detectable. High-molecular-mass (i.e., >100-kDa) proteins, including fibronectin, which may play a role in LPS-mediated PMN priming (4), were recovered in fractions 1 to 7, and medium-molecular-mass (i.e., 50- to 100-kDa) proteins were recovered in fractions 5 to 17. The fractionated plasma was analyzed by SDS-PAGE (data not shown), and individual fractions were used as substitutes for whole plasma in PMN incubation (Fig. 2). Under this condition, it was observed that the plasma factors necessary for LPS activation of p38 in PMN were present in fractions 5 to 16 (Fig. 2), in which there were mainly proteins in the 50- to 100-kDa range.
FIG. 2.
Size fractionation of the plasma factor(s) that supports LPS activation of p38. PMN were incubated in H-medium supplemented with 5% whole or fractionated (on a 30-ml Sephacryl S-200 column) plasma in the absence or presence of 5 ng of LPS/ml at 37°C for 20 min. Cellular proteins were prepared and analyzed for p38 activation as described in the legend to Fig. 1.
Since the plasma factors that enabled LPS to activate p38 in human PMN were present in fractions with molecular masses of 50 to 100 kDa, we speculated that LBP (a 68-kDa plasma protein) was the factor supporting LPS-PMN interaction. We tested this possibility by using purified human LBP as a plasma substitute and by pretreating plasma with an anti-LBP MAb to neutralize the LBP in plasma before it was added to the cells.
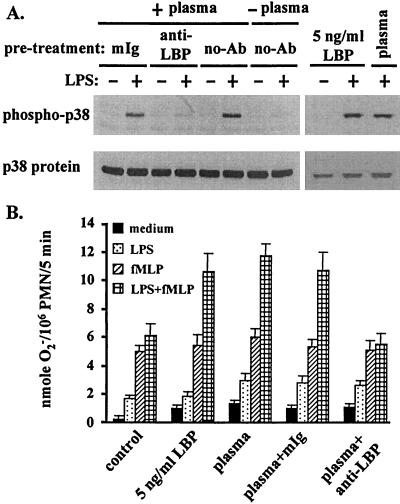
In PMN incubated with plasma which was pretreated with the anti-LPB MAb (at 20 μg/ml for 1 h at room temperature), LPS failed to induce p38 activation (Fig. 3A) or to prime the fMLP-stimulated respiratory burst (Fig. 3B). Furthermore, purified LBP (5 ng/ml) acted as a substitute for plasma in stimulating the PMN response to LPS-induced p38 activation (Fig. 3A) and priming the fMLP-induced respiratory burst (Fig. 3B).
FIG. 3.
LBP is the plasma factor that supports LPS activation of p38 and priming of the PMN respiratory burst. (A) PMN were resuspended in H-medium supplemented with 5% plasma pretreated with a control mouse IgG (mIg) or a mouse anti-LBP Ab and were incubated in the absence or presence of 5 ng of LPS/ml at 37°C for 20 min. Some cells were incubated in H-medium supplemented with 5 ng of purified LBP/ml. Cellular proteins were prepared and analyzed for p38 activation as described in the legend to Fig. 1. (B) PMN were resuspended at a density of 106/ml in H-medium supplemented with 80 μM cytochrome c and 5 ng of purified LBP/ml or 5% plasma pretreated with a control mIg or a mouse anti-LBPAb. Cells were incubated in the absence or presence of 5 ng of LPS/ml at 37°C for 20 min and then with fMLP (10−6 M) for another 5 min. The amount of O2− produced by the cells was calculated from the absorbance of the incubation medium at 550 nm.
LPS activation of p38 and priming of respiratory burst in human PMN is CD14 dependent.
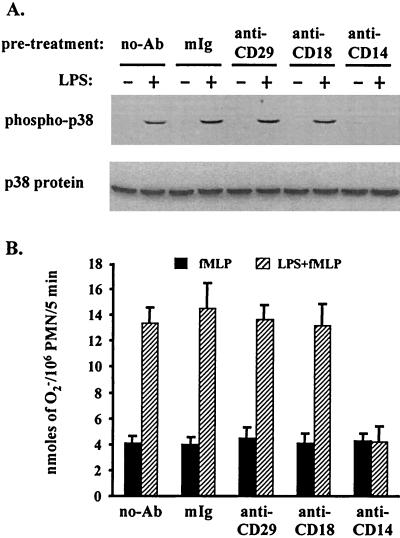
Because the interaction between the LPS-LBP complex and PMN is thought to be mediated by the cell surface receptor CD14 (45, 46), we assessed this and other candidate cell surface receptors involved in the LPS mediated activation of p38 and priming of neutrophil functions. Before treatment with LPS (5 ng/ml), PMN were preincubated with antibodies specific for CD29 (β chain of β1 integrins; MAb 3S3), CD18 (β chain of β2 integrins; MAb IB4), or CD14 (MAb MY4), or with normal mouse IgG as a control. As shown in Fig. 4A, LPS activation of p38 was completely abolished by the CD14-specific antibody. In contrast, antibodies to CD29 and CD18, used either alone (Fig. 4A) or in combination (data not shown), did not affect the action of LPS. Moreover, CD14-dependent LPS activation of p38 was highly coincident with LPS priming of the fMLP-induced respiratory burst, which also appeared to be CD14 dependent (Fig. 4B).
FIG. 4.
CD14, but not β integrins, mediates the LPS activation of p38 and priming of the respiratory burst. PMN either were not pretreated or were pretreated with either control mouse IgG (mIg) or a mouse anti-CD29, anti-CD18, or anti-CD14 Ab for 1 h at 4°C. Cells were then incubated with 5% plasma with or without LPS at 5 ng/ml and were analyzed for activation of p38 (A) or production of O2− (B) as described in the legends to Fig. 1 and 3.
p38 is required for LPS priming of PMN respiratory burst.
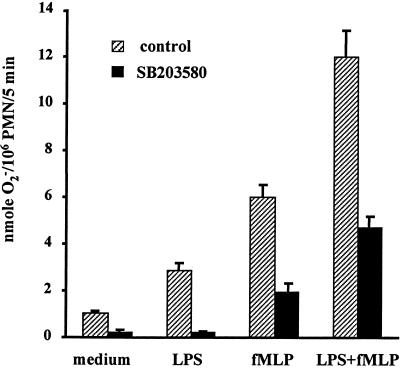
Next we examined the involvement of p38 in LPS priming of the PMN oxidative burst by using the p38-specific inhibitor SB203580. As shown in Fig. 5, in PMN without inhibitor, LPS alone (25 min) induced a slightly increased production of O2− (from 1.0 ± 0.1 to 2.9 ± 0.3 nmol/106 PMN; P < 0.05). On the other hand, fMLP alone (at 10−6 M for 5 min) triggered a moderate increase (from 1.0 ± 0.1 to 6.0 ± 0.6 nmol/106 PMN/5 min; P < 0.01). In PMN primed with LPS for 20 min, fMLP stimulated a doubled increase of O2− release (from 6.0 ± 0.6 to 12.0 ± 1.2 nmol/106 PMN/5 min; P < 0.01). However, when cells were pretreated with the p38 inhibitor SB203580 (at 5 μM, a concentration which effectively abolished p38 activation by LPS [data not shown]), the fMLP-induced production of O2− by LPS-primed PMN decreased to only 4.7 ± 0.5 from 12.0 ± 1.2 nmol/106 PMN (P < 0.01) (Fig. 5). SB203580 treatment also attenuated the generation of O2− by unstimulated PMN as well as by those stimulated with either LPS or fMLP alone (Fig. 5).
FIG. 5.
p38 activation is partially required for LPS priming of the PMN respiratory burst. PMN in H-medium supplemented with 5% plasma were pretreated or not with 5 μM SB203580 for 10 min at room temperature and then assayed for O2− production as described in the legend to Fig. 3.
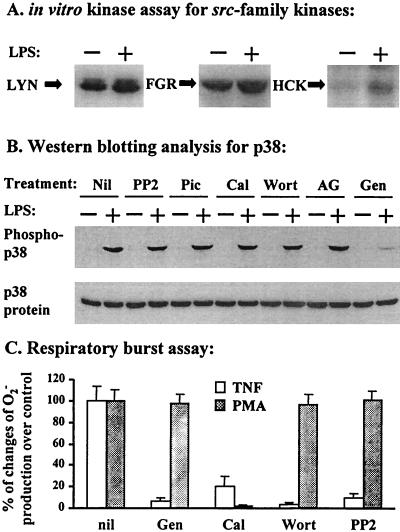
LPS activates Src-family kinases in human PMN, but they are not required for p38 activation.
In order to identify the mediator(s) that functionally links CD14 to p38, we assayed the kinase activities of Src-family tyrosine kinases in PMN treated with LPS, because these enzymes have been implicated in PMN receptor signaling (2). Under our experimental conditions, enhanced kinase activity was detected for all three members of the Src-like kinases (p53/56lyn, p58fgr, and p59hck) expressed in human PMN when cells were stimulated with LPS (5 ng/ml) plus 5% plasma for 20 min (Fig. 6A). Nevertheless, treatment of PMN with the Src-family kinase-specific inhibitor PP2 did not affect the LPS activation of p38 in human PMN (Fig. 6B), suggesting that these kinases are highly unlikely as upstream mediators for p38 activation. p72syk, the protein kinase C (PKC) family, and phosphatidylinositol 3-kinase (PI 3-kinase) are also unlikely to be the link between CD14 and p38, because specific inhibitors for these kinases, i.e., piceatannol, calphostin C, and wortmannin, respectively, did not interfere with LPS activation of p38 (Fig. 6B). However, these inhibitors effectively attenuated the TNF-α-induced PMN respiratory burst (Fig. 6C), for which activities of protein tyrosine kinases, PKC, and PI 3-kinase are necessary (47). The tyrosine kinase inhibitor tyrphostin AG126 has been shown to inhibit the LPS-induced activation of ERKs and the release of nitric oxide from macrophages (26), but it showed no inhibitory effect on p38 activation in LPS-stimulated PMN (Fig. 6B). Overall, we found that only genistein, a tyrosine kinase inhibitor with broad activity, inhibited LPS-induced p38 activation in human PMN (Fig. 6B). This effect of genistein was not due to its cytotoxicity, because the phorbol myristate acetate (PMA)-induced respiratory burst, which is primarily dependent on PKC activity, was not affected by genistein at the concentration used in this study.
FIG. 6.
p38 activation in LPS-treated human PMN is mediated by a genistein-sensitive, tyrphostin AG126-insensitive signaling molecule(s) that does not belong to families of Src, Syk, PKC, and PI 3-kinase. (A) PMN were incubated in H-medium supplemented with 5% plasma with or without LPS at 5 ng/ml and were lysed with RIPA buffer as described in the legend to Fig. 1. The activity of Src-family kinases (Lyn, Fgr, and Hck) was analyzed by an in vitro immunocomplex kinase assay as described in Materials and Methods. (B) PMN were pretreated with either solvent (Nil), 5 μM PP2, 5 μM piceatannol (Pic), 1 μM calphostin C (Cal), 100 nM wortmannin (Wort), 50 μM tyrphostin AG126 (AG), or 50 μM genistein (Gen) for 10 min at room temperature. Cells were then incubated in the presence of 5% plasma with or without LPS at 5 ng/ml, lysed, and analyzed for p38 activation as described in the legend to Fig. 1. (C) PMN were treated with the indicated inhibitors as described above and assayed for O2− production as described in the legend to Fig. 3 by using TNF-α (20 ng/ml) and PMA (10 ng/ml) as stimuli (for 30 min at 37°C). Results are expressed as means ± standard errors of the means (n = 3) of percentages of changes in O2− production from that in the non-inhibitor-treated control (nil).
DISCUSSION
Interactions between LPS and host cells are complex. For example, in the presence of plasma or serum, LPS at 1 to 5 ng per ml (i.e., levels found in the plasma of patients with endotoxemia) does not activate PMN in vitro. However, PMN are primed under these conditions, i.e., they acquire the ability to generate extremely high levels of reactive oxygen radicals when triggered by a second stimulus (11). LBP, CD14, protein tyrosine kinases, and MAP kinases are thought to be important in the LPS-PMN interaction (5, 9, 13, 23-25, 45, 46); however, the relationship between these factors remains to be delineated.
In the present study we observed that LPS induced tyrosine phosphorylation of a 38-kDa protein coincident with the activation of p38. Activation of p38 correlated with LPS priming of PMN, indicating an important role for p38 in this process. This was confirmed by using the p38-specific inhibitor SB203580, which attenuated the LPS enhancement of fMLP-induced O2− release.
The major cell surface receptor for LPS on PMN is CD14, a 55-kDa glycosylphosphatidylinositol-linked membrane protein (46). However, β2 integrin (CD11b/CD18) (45), l-selectin (20), and, more recently, the transmembrane protein Toll-like receptor (16, 40) have also been implicated in LPS signaling. A pivotal role for CD14 in the interaction between PMN and LPS or other bacterial components from both the gram-negative and gram-positive classes has been clearly demonstrated (8, 15, 28-30, 33, 41, 44). CD14 is required for LPS stimulation of a variety of biochemical and cellular functional changes, including activation of phospholipases (39), protein kinase A and PKC (17, 35), protein tyrosine kinases (17, 38), and MAP kinases (Fig. 4A) (3, 9, 23-25). These signaling events are associated with PMN priming (5, 13). As demonstrated in the present study, activation of p38 is also associated with PMN priming.
LPS-CD14 interaction is greatly enhanced in the presence of plasma (45, 46). Two proteins, LBP and fibronectin, have been implicated as factors of plasma needed for efficient PMN priming (4). Therefore, we investigated the role of plasma proteins in LPS activation of p38 in PMN. We found that plasma fractions supporting LPS activation of p38 in human PMN contained predominantly proteins of 50 to 100 kDa, implicating LBP, which has a molecular mass of 68 kDa (46). However, these fractions contain many proteins, so a role for other factors could not be ruled out. In this report, we show that both activation of p38 (Fig. 3A) and priming of PMN (Fig. 3B) by LPS are accomplished by purified LBP and attenuated by treatment of plasma with an anti-LBP MAb.
LPS can induce tyrosine phosphorylation of several cellular proteins, including p38, p44/p42 ERKs, p72syk, JNK, phospholipase D, Pyk2, and Vav, in PMN or other blood leukocytes (5, 7, 13, 14, 17, 24, 25, 38, 43). In the present study we observed that LPS by itself at low concentrations (5 ng/ml) did not change total protein tyrosine phosphorylation in human PMN. However, in the presence of plasma (5%), LPS induced a greatly enhanced phosphorylation of a 38-kDa protein(s) (Fig. 1) which coincided with the phosphorylation of p38. This finding, together with other data presented in this study, suggests an important role of p38 in LPS priming of PMN function. Thus, we speculate that inhibitors targeted to p38 may alter PMN-initiated symptoms for patients with endotoxemia. Nevertheless, it should also be noted that treatment of PMN with SB203580 did not completely block the O2− production induced by LPS or fMLP alone, or by fMLP following LPS priming (Fig. 5), indicating that besides p38, other signaling mechanisms such as ERKs (3) may also operate in these processes.
Tyrosine kinases are thought to mediate LPS-CD14 signaling, since their inhibitors abolish LPS-induced cellular responses including the priming of the PMN respiratory burst (5, 13). Human PMN express three members of Src-family tyrosine kinases, namely, p59hck, p58fgr, and p53/56lyn, that serve as important signaling mediators for PMN responses to a variety of extracellular stimuli (2). In human monocytes, the activity of Src-like kinases has been implicated in CD14-mediated biological effects initiated by LPS (38). As observed in the present study, all three of the Src-like kinases were activated by LPS in human PMN (Fig. 6A). However, these kinases were not required for LPS activation of p38, which was unaffected by the inhibition of these enzymes by their specific inhibitor PP2 at inhibitory concentrations (32) (Fig. 6B and C). Therefore, human PMN may be similar to murine macrophages, in which LPS-induced activation and signaling occur in the absence of Hck, Fgr, and Lyn (21). Although PKC, p72syk, and PI 3-kinase are important mediators required for full activation of the PMN respiratory burst (22, 47), inhibitors of these enzymes failed to abolish this response, making them unlikely upstream activators of p38 following CD14 ligation (Fig. 6B). ERK1 and ERK2 are other candidates for LPS-induced priming in PMN. The growth factor-like receptor tyrosine kinase inhibitor tyrphostin AG126 can block some LPS-induced cellular responses, including activation of ERK in murine macrophages (26). In human PMN, however, it failed to inhibit the activation of p38 by LPS. On the other hand, genistein, a broad tyrosine kinase inhibitor which abolishes LPS priming of PMN (18, 19, 31), markedly attenuated the activation of p38 by LPS in our experiments (Fig. 6B).
These results greatly decrease the number of possible candidates that may link CD14 to the p38 pathway in PMN. Contenders for this role should be functionally genistein sensitive, tyrphostin AG126 insensitive, and unlikely to belong to Src- or Syk-family kinases, PKC, or PI 3-kinase. Defining this mediator(s) will help delineate the mechanisms involved in LPS priming of PMN function and enhance clinical understanding of endotoxemia in gram-negative bacterial sepsis.
Acknowledgments
This work was supported by grants MT. 14439 and MT. 15282 from the Medical Research Council of Canada. S. R. Yan and W. Al-Hertani are supported by awards from the IWK Health Centre.
We thank G. Berton, Institute of General Pathology, University of Verona, Verona, Italy, for kindly providing the anti-p58fgr Ab and Pam Kirby for laboratory and secretarial support.
Editor: R. N. Moore
REFERENCES
- 1.Bannerman, D. D., and S. E. Goldblum. 1999. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab. Investig. 79:1181-1199. [PubMed] [Google Scholar]
- 2.Berton, G., S. R. Yan, L. Fumagalli, and C. A. Lowell. 1996. Neutrophil activation by adhesion: mechanisms and pathophysiological implications. Int. J. Clin. Lab. Res. 26:160-177. [DOI] [PubMed] [Google Scholar]
- 3.Bonner, S., S. R. Yan, D. M. Byers, and R. Bortolussi. 2001. Activation of extracellular signal-related protein kinases 1 and 2 of the mitogen-activated protein kinase family by lipopolysaccharide requires plasma in neutrophils from adults and newborns. Infect. Immun. 69:3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortolussi, R., K. Rajaraman, G. Qing, and R. Rajaraman. 1997. Fibronectin enhances in vitro lipopolysaccharide priming of polymorphonuclear leukocytes. Blood 89:4182-4189. [PubMed] [Google Scholar]
- 5.Coffer, P. J., and L. Koenderman. 1997. Granulocyte signal transduction and priming: cause without effect? Immunol. Lett. 57:27-31. [DOI] [PubMed] [Google Scholar]
- 6.Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 7.Detmers, P. A., D. Zhou, E. Polizzi, R. Thieringer, W. A. Hanlon, S. Vaidya, and V. Bansal. 1998. Role of stress-activated mitogen-activated protein kinase (p38) in β2-integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J. Immunol. 161:1921-1929. [PubMed] [Google Scholar]
- 8.Ferrero, E., D. Jiao, B. Z. Tsuberi, L. Tesio, G. W. Rong, A. Haziot, and S. M. Goyert. 1993. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc. Natl. Acad. Sci. USA 90:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geppert, T. D., C. E. Whitehurst, P. Thompson, and B. Beutler. 1994. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol. Med. 1:93-103. [PMC free article] [PubMed] [Google Scholar]
- 10.Giroir, B. P., P. A. Quint, P. Barton, E. A. Kirsch, L. Kitchen, B. Goldstein, B. J. Nelson, N. I. Wedel, S. F. Carroll, and P. J. Scannon. 1997. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet 350:1439-1443. [DOI] [PubMed] [Google Scholar]
- 11.Guthrie, L. A., L. C. McPhail, P. M. Henson, and R. B. Johnston, Jr. 1984. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J. Exp. Med. 160:1656-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale, K. K., D. Trollinger, M. Rihanek, and C. L. Manthey. 1999. Differential expression and activation of p38 mitogen-activated protein kinase α, β, γ, and δ in inflammatory cell lineages. J. Immunol. 162:4246-4252. [PubMed] [Google Scholar]
- 13.Hallett, M. B., and D. Lloyds. 1995. Neutrophil priming: the cellular signals that say "amber' but not "green.' Immunol. Today 16:264-268. [DOI] [PubMed] [Google Scholar]
- 14.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haziot, A., E. Ferrero, F. Kontgen, N. Hijiya, S. Yamamoto, J. Silver, C. L. Stewart, and S. M. Goyert. 1996. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4:407-414. [DOI] [PubMed] [Google Scholar]
- 16.Henneke, P., and D. T. Golenbock. 2001. TIRAP: how Toll receptors fraternize. Nat. Immunol. 2:828-830. [DOI] [PubMed] [Google Scholar]
- 17.Herrera-Velit, P., and N. E. Reiner. 1996. Bacterial lipopolysaccharide induces the association and coordinate activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J. Immunol. 156:1157-1165. [PubMed] [Google Scholar]
- 18.Lloyds, D., and M. B. Hallett. 1994. Neutrophil “priming” induced by orthovanadate: evidence of a role for tyrosine phosphorylation. Biochem. Pharmacol. 48:15-21. [DOI] [PubMed] [Google Scholar]
- 19.Lloyds, D., N. P. J. Brindle, and M. B. Hallett. 1995. Priming of human neutrophils by tumour necrosis factor-α and substance P is associated with tyrosine phosphorylation. Immunology 84:220-226. [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra, R., and M. I. Bird. 1997. l-Selectin: a novel receptor for lipopolysaccharide and its potential role in bacterial sepsis. Bioessays 19:919-923. [DOI] [PubMed] [Google Scholar]
- 21.Meng, F., and C. A. Lowell. 1997. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 185:1661-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocsai, A., Z. Jakus, T. Vantus, G. Berton, C. A. Lowell, and E. Ligeti. 2000. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J. Immunol. 164:4321-4331. [DOI] [PubMed] [Google Scholar]
- 23.Nahas, N., T. F. Molski, G. A. Fernandez, and R. I. Sha'afi. 1996. Tyrosine phosphorylation and activation of a new mitogen-activated protein (MAP)-kinase cascade in human neutrophils stimulated with various agonists. Biochem. J. 318:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nick, J. A., N. J. Avdi, S. K. Young, L. A. Lehman, P. P. McDonald, S. C. Frasch, M. A. Billstrom, P. M. Henson, G. L. Johnson, and G. S. Worthen. 1999. Selective activation and functional significance of p38α mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J. Clin. Investig. 103:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nick, J. A., N. J. Avdi, P. Gerwins, G. L. Johnson, and G. S. Worthen. 1996. Activation of a p38 mitogen-activated protein kinase in human neutrophils by lipopolysaccharide. J. Immunol. 156:4867-4875. [PubMed] [Google Scholar]
- 26.Novogrodsky, A., A. Vanichkin, M. Patya, A. Gazit, N. Osherov, and A. Levitzki. 1994. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science 264:1319-1322. [DOI] [PubMed] [Google Scholar]
- 27.Parrillo, J. E., M. M. Parker, C. Natanson, A. F. Suffredini, R. L. Danner, R. E. Cunnion, and F. P. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113:227-242. [DOI] [PubMed] [Google Scholar]
- 28.Perera, P. Y., S. N. Vogel, G. R. Detore, A. Haziot, and S. M. Goyert. 1997. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J. Immunol. 158:4422-4429. [PubMed] [Google Scholar]
- 29.Pugin, J., C. C. Schurer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugin, J., I. D. Heumann, A. Tomasz, V. V. Kravchenko, Y. Akamatsu, M. Nishijima, M. P. Glauser, P. S. Tobias, and R. J. Ulevitch. 1994. CD14 is a pattern recognition receptor. Immunity 1:509-516. [DOI] [PubMed] [Google Scholar]
- 31.Rafiee, P., J. K. Lee, C.-C. Leung, and T. A. Raffin. 1995. TNF-α induces human neutrophils. J. Immunol. 154:4785-4792. [PubMed] [Google Scholar]
- 32.Salazar, E. P., and E. Rozengurt. 1999. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 274:28371-28378. [DOI] [PubMed] [Google Scholar]
- 33.Schimke, J., J. Mathison, J. Morgiewicz, and R. J. Ulevitch. 1998. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc. Natl. Acad. Sci. USA 95:13875-13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schopf, R. E., R. Keller, M. Rehder, P. Benes, F. Kallinowski, and P. Vaupel. 1990. TNF-α primes polymorphonuclear leukocytes for an enhanced respiratory burst to a similar extent as bacterial lipopolysaccharide. J. Investig. Dermatol. 95:216S-218S. [DOI] [PubMed] [Google Scholar]
- 35.Shapira, L., S. Takashiba, C. Champagne, S. Amar, and T. E. Van Dyke. 1994. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-α and IL-1β production by human monocytes. J. Immunol. 153:1818-1824. [PubMed] [Google Scholar]
- 36.Smith, J. A. 1994. Neutrophils, host defence, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686. [DOI] [PubMed] [Google Scholar]
- 37.Smolen, J. E., T. K. Petersen, C. Koch, S. J. O'Keefe, W. A. Hanlon, S. Seo, D. Pearson, M. C. Fossett, and S. I. Simon. 2000. l-Selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinase. J. Biol. Chem. 275:15876-15884. [DOI] [PubMed] [Google Scholar]
- 38.Stefanova, I., M. L. Corcoran, E. M. Horak, L. M. Wahl, J. B. Bolen, and I. D. Horak. 1993. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J. Biol. Chem. 268:20725-20728. [PubMed] [Google Scholar]
- 39.Syrbu, S. I., W. H. Waterman, T. F. Molski, D. Nagarkatti, J. J. Hajjar, and R. I. Sha'afi. 1999. Phosphorylation of cytosolic phospholipase A2 and the release of arachidonic acid in human neutrophils. J. Immunol. 162:2334-2340. [PubMed] [Google Scholar]
- 40.Ulevitch, R. J. 1999. Endotoxin opens the Toll gates to innate immunity. Nat. Med. 5:144-145. [DOI] [PubMed] [Google Scholar]
- 41.Verbon, A., P. E. Dekkers, T. ten Hove, C. E. Hack, J. P. Pribble, T. Turner, S. Souza, T. Axtelle, F. J. Hoek, S. J. van Deventer, and T. van der Poll. 2001. IC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humans. J. Immunol. 166:3599-3605. [DOI] [PubMed] [Google Scholar]
- 42.Waage, A., P. Brandtzaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J. Exp. Med. 169:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams, L. M., and A. J. Ridley. 2000. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J. Immunol. 164:2028-2036. [DOI] [PubMed] [Google Scholar]
- 44.Wright, S. D. 1995. CD14 and innate recognition of bacteria. J. Immunol. 155:6-8. [PubMed] [Google Scholar]
- 45.Wright, S. D., R. A. Ramos, A. Hermanowski-Vosatka, P. Rockwell, and P. A. Detmers. 1991. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J. Exp. Med. 173:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 47.Yan, S. R., and M. J. Novak. 1999. Diverse effects of neutrophil integrin occupation on respiratory burst activation. Cell. Immunol. 195:119-126. [DOI] [PubMed] [Google Scholar]
- 48.Yan, S. R., L. Fumagalli, and G. Berton. 1995. Activation of p58c-fgr and p53/56lyn in adherent human neutrophils: evidence for a role of divalent cations in regulating neutrophil adhesion and protein tyrosine kinase activation. J. Inflamm. 145:297-311. [PubMed] [Google Scholar]
- 49.Zu, Y. L., J. Qi, A. Gilchrist, G. A. Fernandez, D. Vazquez-Abad, D. L. Kreutzer, C. K. Huang, and R. I. Sha'afi. 1998. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-α or FMLP stimulation. J. Immunol. 160:1982-1989. [PubMed] [Google Scholar]