Abstract
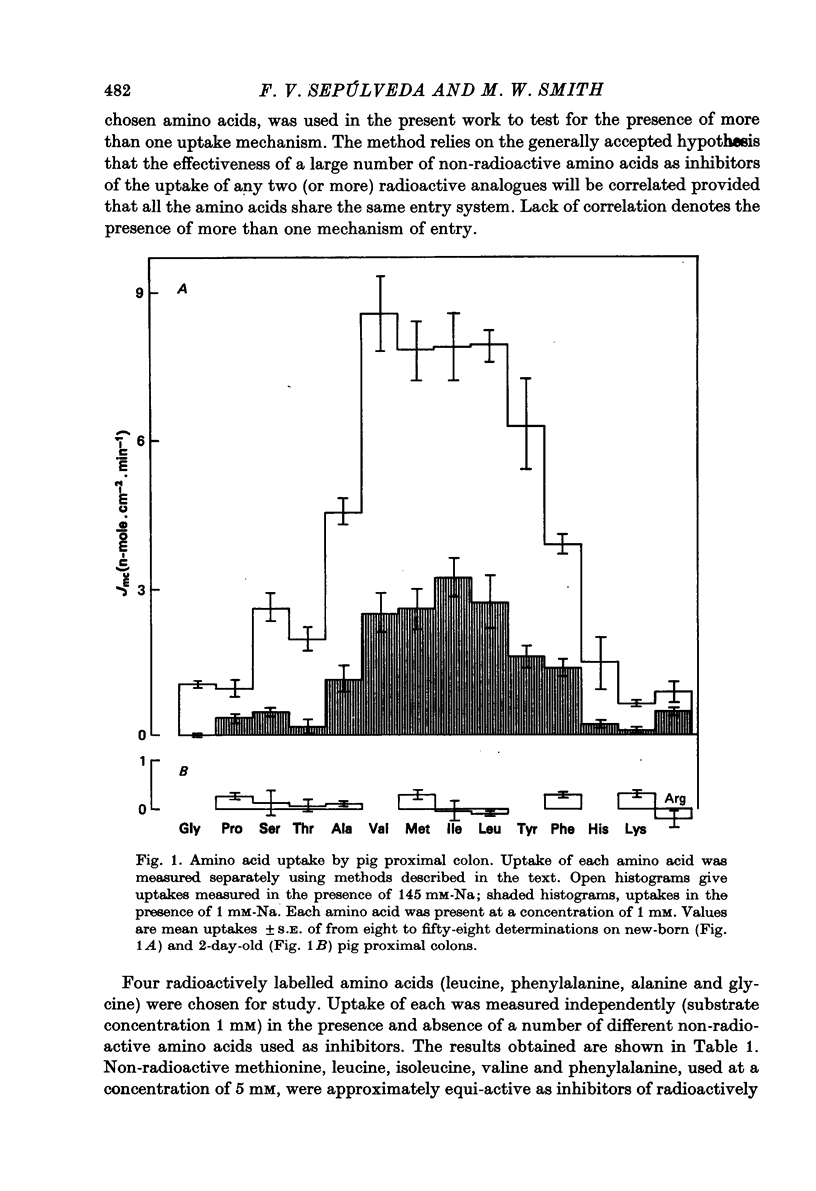
1. Mucosal amino acid uptake by pig proximal colon, measured independently for fourteen different amino acids each used at a concentration of 1 mM, ranged from 0·6 to 8·6 n-mole. cm-2. min-1 in the new-born to 0 to 0·3 n-mole. cm-2. min-1 in the 2-day-old animal. Long chain amino acids entered the mucosa of new-born pig proximal colon much more readily than did short chain amino acids.
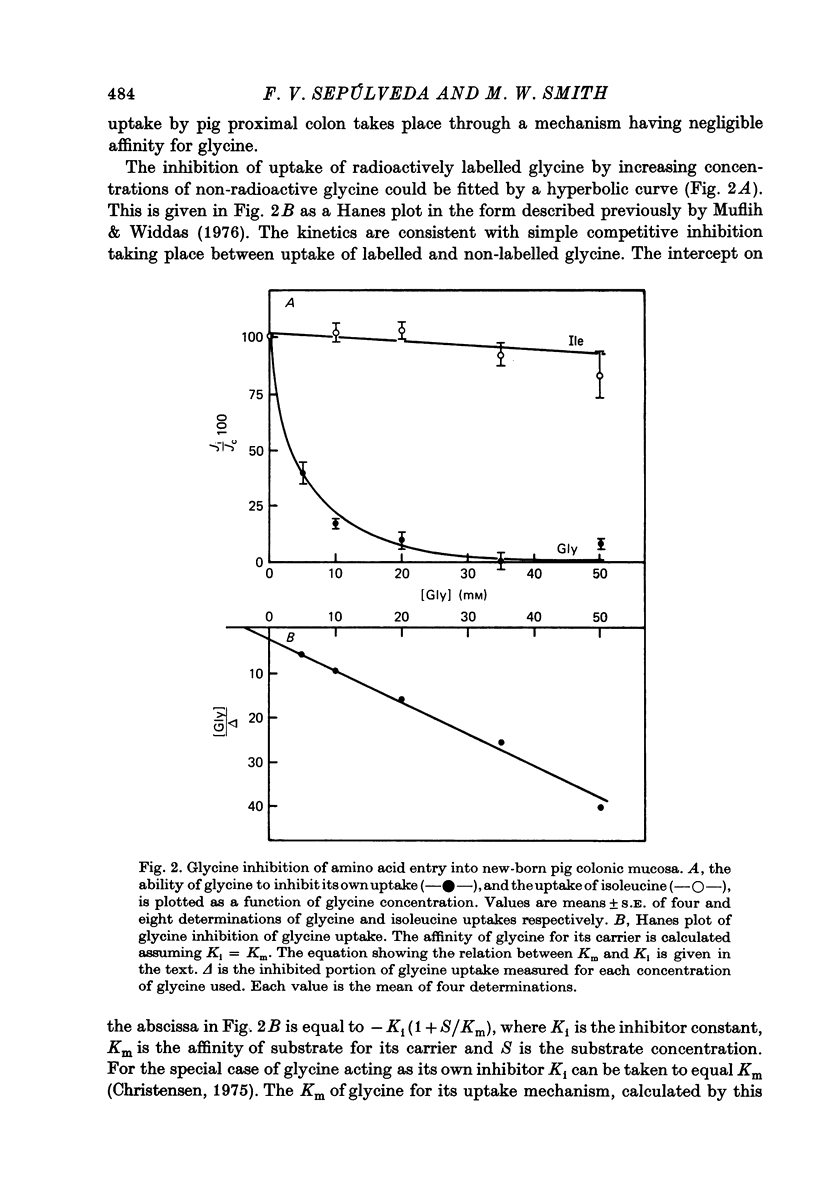
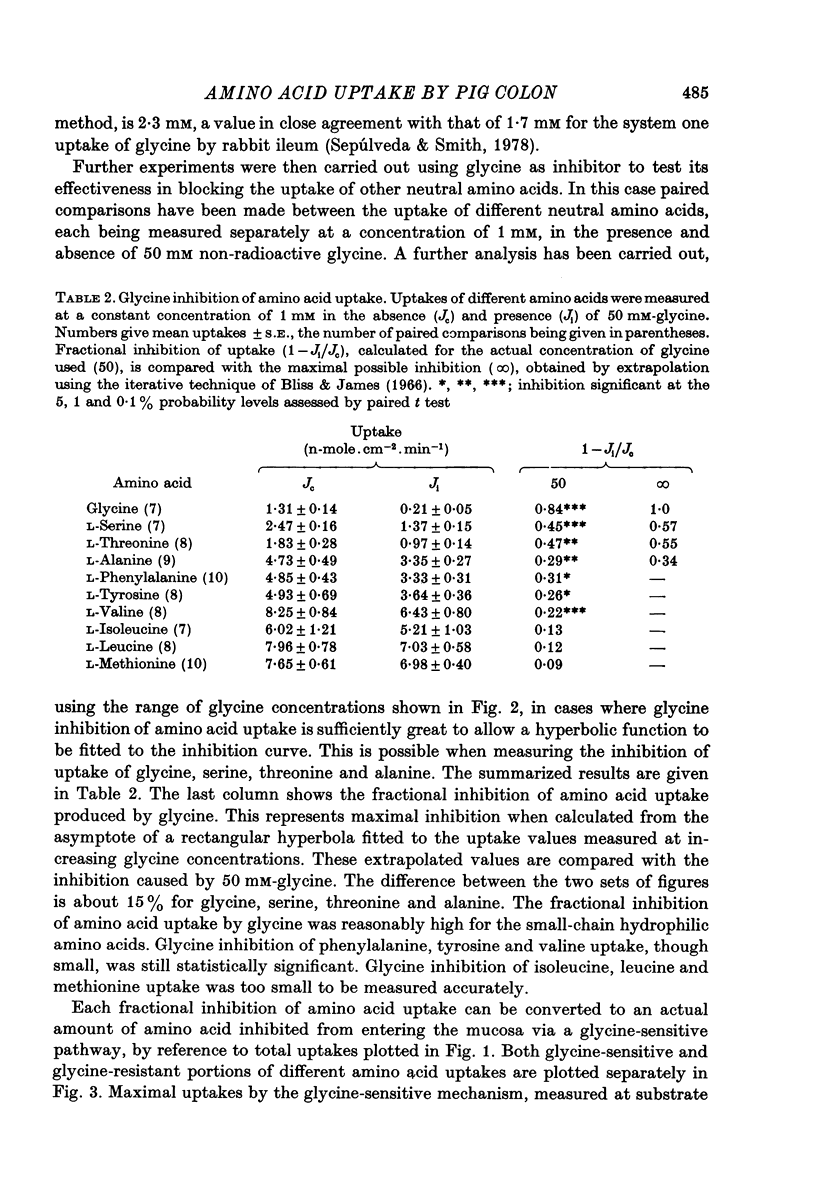
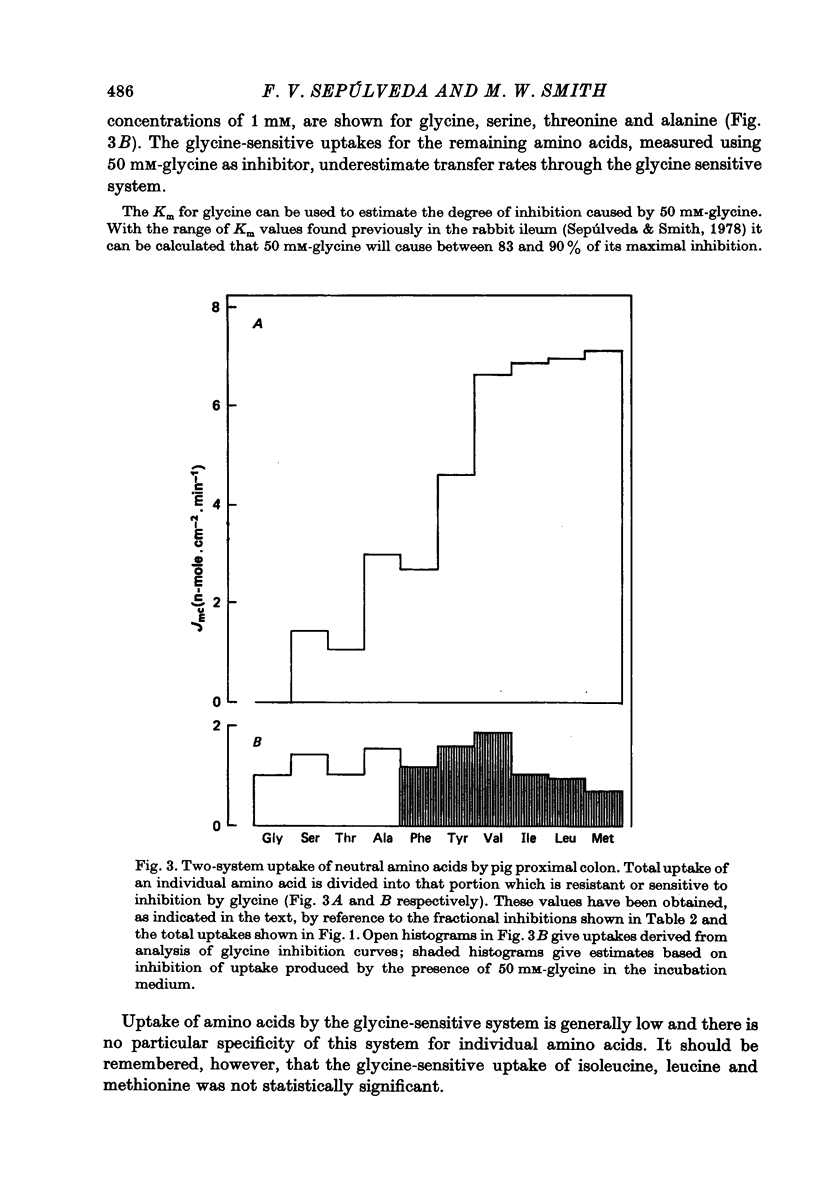
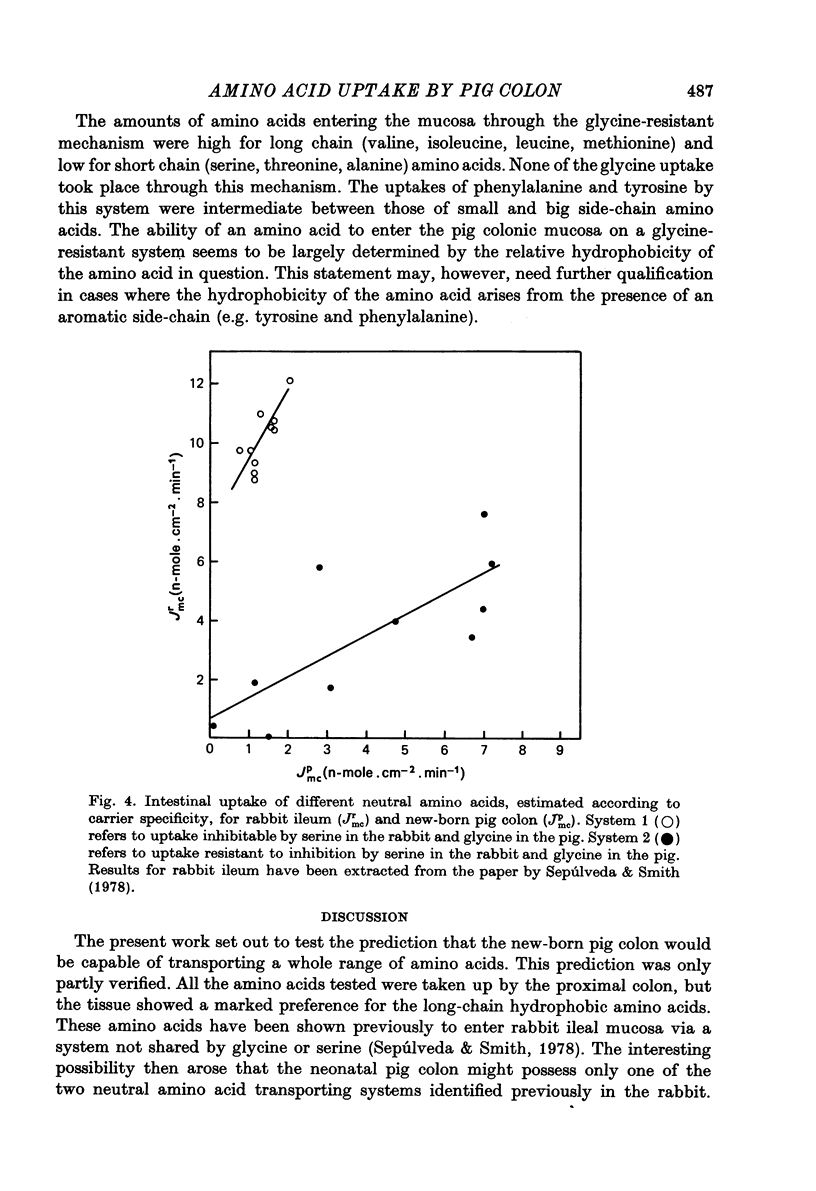
2. Glycine was used extensively to inhibit the uptake of other neutral amino acids. The degree of maximal inhibition produced depended on the amino acid used. The relative inability of glycine to inhibit the uptake of long chain amino acids suggested that these compounds could cross the brush border on a carrier inaccessible to glycine. The glycine-sensitive uptake remained more or less constant for all amino acids tested (1-2 n-mole.cm-2.min-1); the glycine-insensitive uptake varied from 0 to 7 n-mole.cm-2.min-1 (glycine and methionine respectively).
3. It is suggested that at least two mechanisms exist for the entry of neutral amino acids into pig proximal colon, one showing specificity for hydrophobic amino acids and the other having broad specificity. The mechanism responsible for the uptake of long chain essential amino acids predominates over the less specific mechanism.
4. These results are discussed in relation to previous work carried out on the rabbit ileum where two similar systems for neutral amino acid entry have been shown to be present. Both tissues transport hydrophobic amino acids on their own specific carrier at approximately the same rate; the ability of the pig colon to transport amino acids on the broad specificity carrier is eight times less than in the rabbit ileum. The possibility is raised that this system is subject to regulation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Christensen H. N. Some special kinetic problems of transport. Adv Enzymol Relat Areas Mol Biol. 1969;32:1–20. doi: 10.1002/9780470122778.ch1. [DOI] [PubMed] [Google Scholar]
- Henriques de Jesus C., Smith M. W. Sodium transport by the small intestine of new-born and suckling pigs. J Physiol. 1974 Nov;243(1):211–224. doi: 10.1113/jphysiol.1974.sp010750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénin S., Smith M. W. Electrical properties of pig colonic mucosa measured during early post-natal development. J Physiol. 1976 Oct;262(1):169–187. doi: 10.1113/jphysiol.1976.sp011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P. S., Smith M. W. Methionine transport by pig colonic mucosa measured during early post-natal development. J Physiol. 1976 Oct;262(1):151–168. doi: 10.1113/jphysiol.1976.sp011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P. S., Smith M. W., Wooding F. B. Proceedings: Time-dependent changes in methionine transport across the helicoidal colon of the new-born pig. J Physiol. 1976 May;257(1):38P–39P. [PubMed] [Google Scholar]
- Jarvis L. G., Morgan G., Smith M. W., Wooding F. B. Cell replacement and changing transport function in the neonatal pig colon. J Physiol. 1977 Dec;273(3):717–729. doi: 10.1113/jphysiol.1977.sp012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. H., Zand R. Basis for substrate preference of amino acid transport system L over amino acid transport system A. Biochemistry. 1977 Aug 23;16(17):3820–3824. doi: 10.1021/bi00636a016. [DOI] [PubMed] [Google Scholar]
- Muflih I. W., Widdas W. F. Sugars and sugar derivatives which inhibit the short-circuit current of the everted small intestine of the rat. J Physiol. 1976 Dec;263(2):101–114. doi: 10.1113/jphysiol.1976.sp011623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- OXENDER D. L. STEREOSPECIFICITY OF AMINO ACID TRANSPORT FOR EHRLICH TUMOR CELLS. J Biol Chem. 1965 Jul;240:2976–2982. [PubMed] [Google Scholar]
- Tager H. S., Christensen H. N. Transport of the four isomers of 2-aminonorbornane-2-carboxylic acid in selected mammalian systems and in Escherichia coli. J Biol Chem. 1971 Dec 25;246(24):7572–7580. [PubMed] [Google Scholar]
- Yudilevich D. L., Sépulveda F. V. The specificity of amino acid and sugar carriers in the capillaries of the dog brain studied in vivo by rapid indicator dilution. Adv Exp Med Biol. 1976;69:77–87. doi: 10.1007/978-1-4684-3264-0_6. [DOI] [PubMed] [Google Scholar]


