Abstract
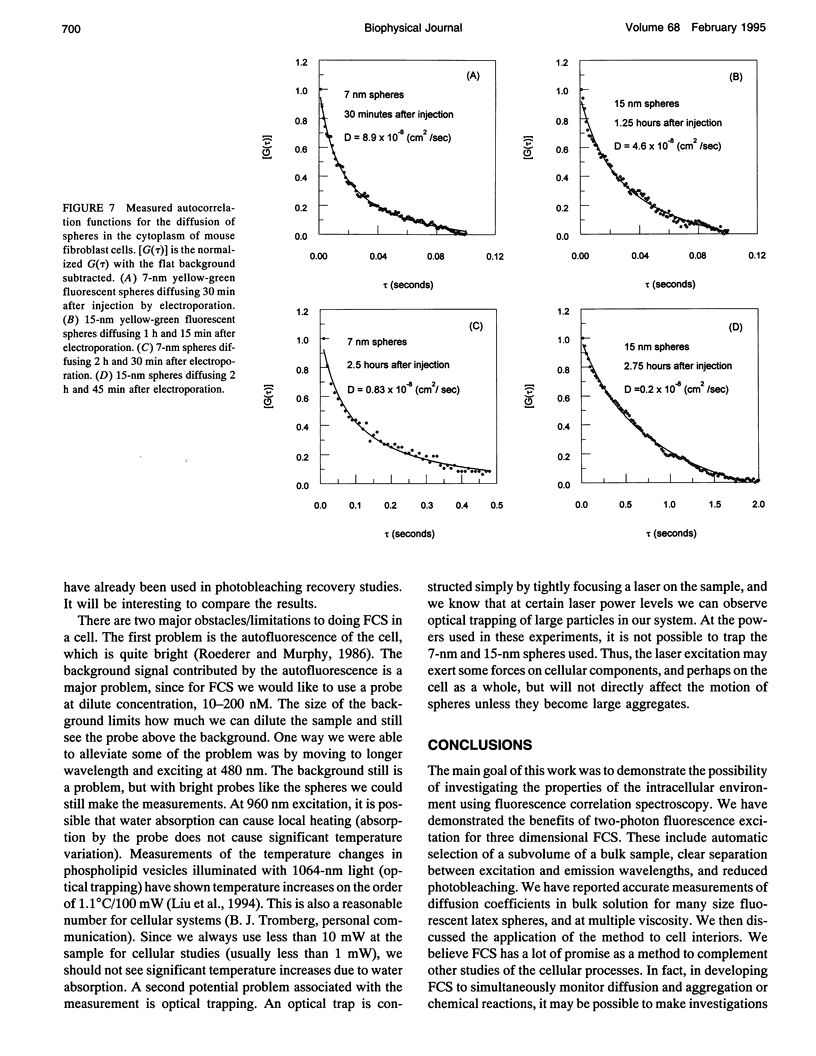
We report on the application of two photon molecular excitation to fluorescence correlation spectroscopy. We demonstrate the first fluorescence correlation spectroscopy measurements of translational mobility in the cytoplasm of living cells. Two-photon excitation inherently excites small sample volumes in three dimensions, providing depth discrimination similar to confocal microscopy, without emission pinholes. We demonstrated accurate measurements of the diffusion constant, D, for particles of several different known sizes, in bulk solutions of different viscosity. We then showed measurements of translational diffusion for 7- and 15-nm radius latex beads in the cytoplasm of mouse fibroblast cells. We measured time-dependent diffusion coefficients. When first injected in the cells, the spheres moved from two to five times slower than in water, with average rates of 18 x 10(-8) cm2/s for the 7 nm and 5 x 10(-8) cm2/s for the 15 nm radius spheres. After a few hours, spheres stick to the cells, and the motion slows down 10 to 100 times.
Full text
PDF
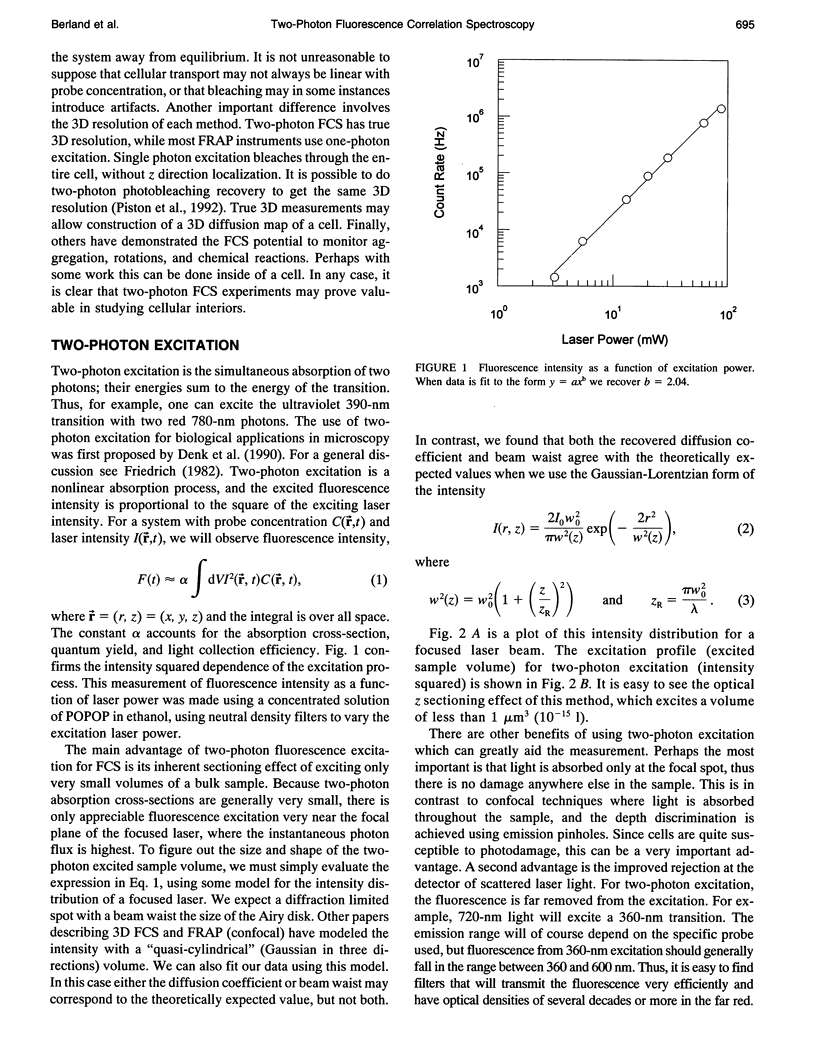
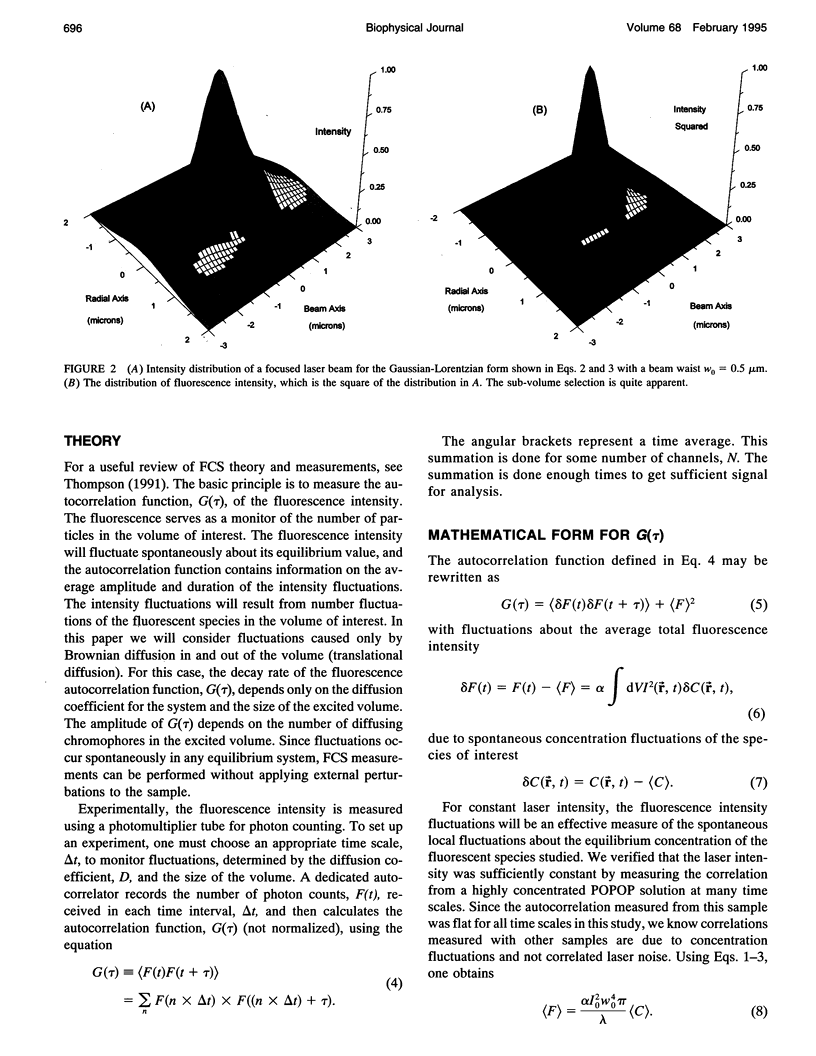

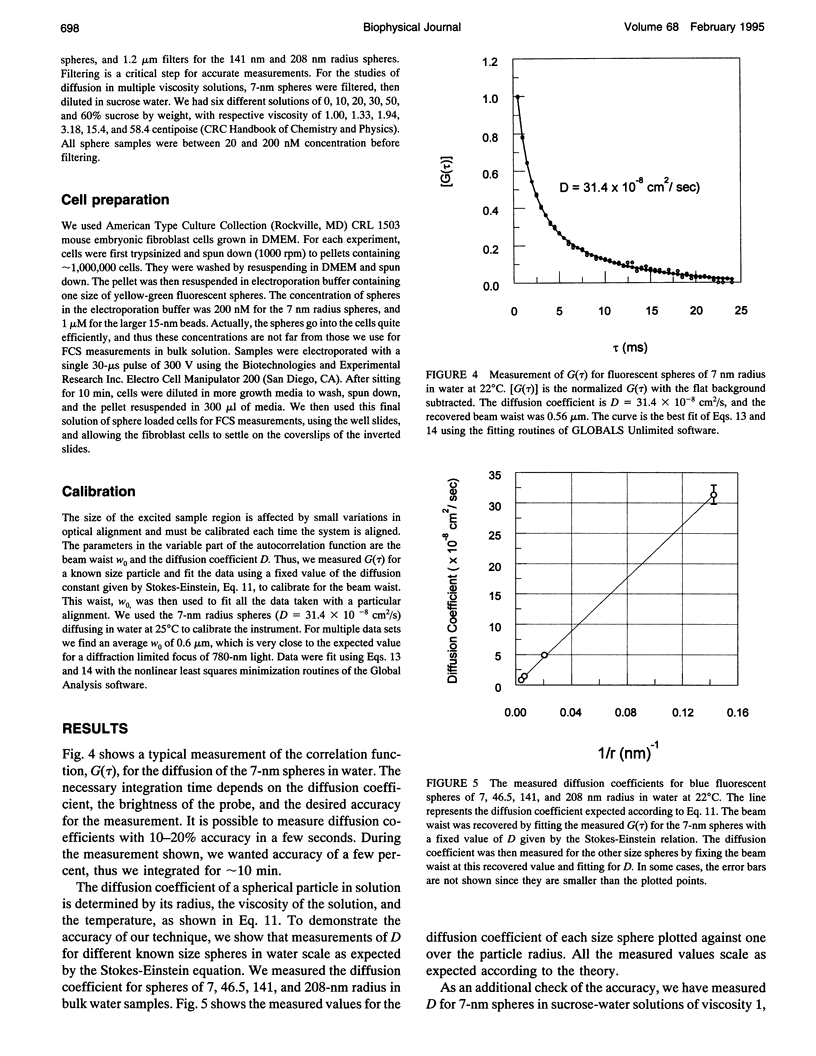
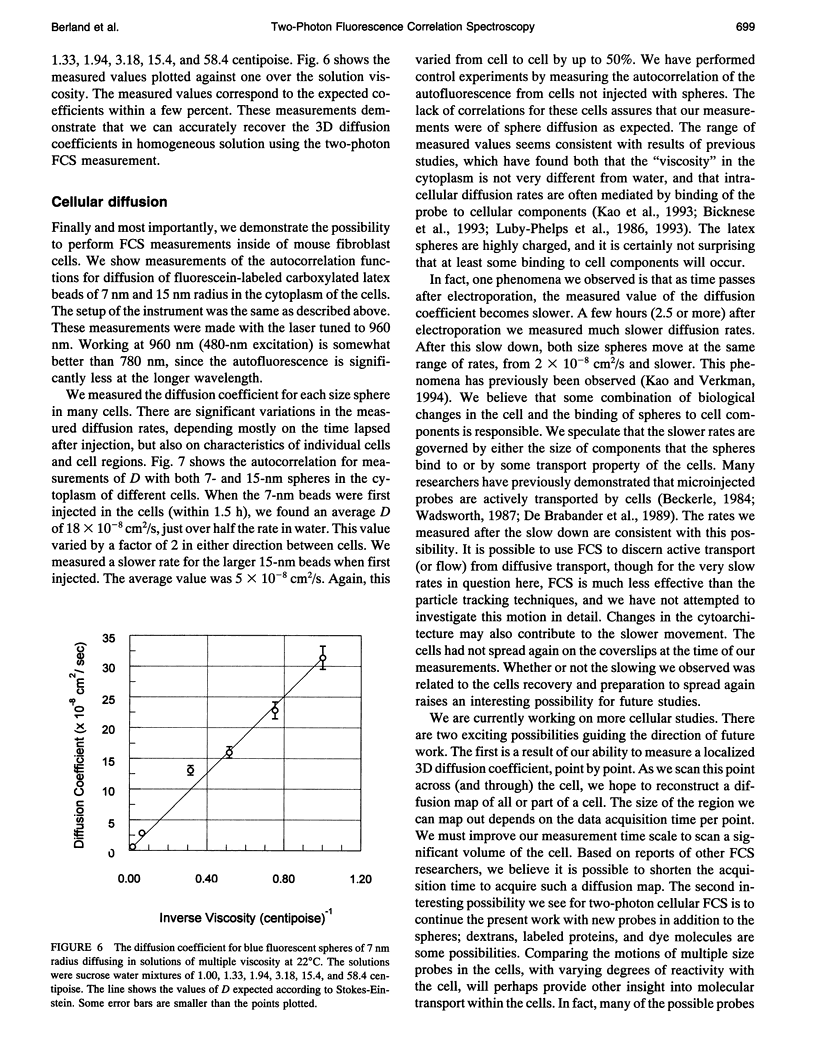

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerle M. C. Microinjected fluorescent polystyrene beads exhibit saltatory motion in tissue culture cells. J Cell Biol. 1984 Jun;98(6):2126–2132. doi: 10.1083/jcb.98.6.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknese S., Periasamy N., Shohet S. B., Verkman A. S. Cytoplasmic viscosity near the cell plasma membrane: measurement by evanescent field frequency-domain microfluorimetry. Biophys J. 1993 Sep;65(3):1272–1282. doi: 10.1016/S0006-3495(93)81179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J. Motion of myosin fragments during actin-activated ATPase: fluorescence correlation spectroscopy study. Biopolymers. 1979 Nov;18(11):2807–2820. doi: 10.1002/bip.1979.360181111. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geerts H., Nuydens R., Nuyens R. Detection of gold probes with video-enhanced contrast microscopy: nanovid microscopy. Am J Anat. 1989 Jun-Jul;185(2-3):282–295. doi: 10.1002/aja.1001850221. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Eigen M., Rigler R. Sorting single molecules: application to diagnostics and evolutionary biotechnology. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5740–5747. doi: 10.1073/pnas.91.13.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Icenogle R. D., Elson E. L. Fluorescence correlation spectroscopy and photobleaching recovery of multiple binding reactions. I. Theory and FCS measurements. Biopolymers. 1983 Aug;22(8):1919–1948. doi: 10.1002/bip.360220808. [DOI] [PubMed] [Google Scholar]
- Icenogle R. D., Elson E. L. Fluorescence correlation spectroscopy and photobleaching recovery of multiple binding reactions. I. Theory and FCS measurements. Biopolymers. 1983 Aug;22(8):1919–1948. doi: 10.1002/bip.360220808. [DOI] [PubMed] [Google Scholar]
- Kao H. P., Abney J. R., Verkman A. S. Determinants of the translational mobility of a small solute in cell cytoplasm. J Cell Biol. 1993 Jan;120(1):175–184. doi: 10.1083/jcb.120.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. P., Verkman A. S. Tracking of single fluorescent particles in three dimensions: use of cylindrical optics to encode particle position. Biophys J. 1994 Sep;67(3):1291–1300. doi: 10.1016/S0006-3495(94)80601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Morgan F., Cowan A. E., Carson J. H. Scanning concentration correlation spectroscopy using the confocal laser microscope. Biophys J. 1994 Feb;66(2 Pt 1):502–507. doi: 10.1016/s0006-3495(94)80801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Mujumdar S., Mujumdar R. B., Ernst L. A., Galbraith W., Waggoner A. S. A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys J. 1993 Jul;65(1):236–242. doi: 10.1016/S0006-3495(93)81075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994 Feb;6(1):3–9. doi: 10.1016/0955-0674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Meyer T., Schindler H. Particle counting by fluorescence correlation spectroscopy. Simultaneous measurement of aggregation and diffusion of molecules in solutions and in membranes. Biophys J. 1988 Dec;54(6):983–993. doi: 10.1016/S0006-3495(88)83036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. Molecular aggregation characterized by high order autocorrelation in fluorescence correlation spectroscopy. Biophys J. 1987 Aug;52(2):257–270. doi: 10.1016/S0006-3495(87)83213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O. Diffusion and aggregation in biological membranes. Can J Biochem Cell Biol. 1984 Nov;62(11):1158–1166. doi: 10.1139/o84-149. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., Höddelius P. L., Wiseman P. W., Seger O., Magnusson K. E. Quantitation of membrane receptor distributions by image correlation spectroscopy: concept and application. Biophys J. 1993 Sep;65(3):1135–1146. doi: 10.1016/S0006-3495(93)81173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O. Scanning fluorescence correlation spectroscopy. I. Theory and simulation of aggregation measurements. Biophys J. 1986 Apr;49(4):809–815. doi: 10.1016/S0006-3495(86)83709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O. Scanning fluorescence correlation spectroscopy. I. Theory and simulation of aggregation measurements. Biophys J. 1986 Apr;49(4):809–815. doi: 10.1016/S0006-3495(86)83709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Elson E. L. Distribution of molecular aggregation by analysis of fluctuation moments. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5479–5483. doi: 10.1073/pnas.87.14.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre P. R., Petersen N. O. Relative ligand binding to small or large aggregates measured by scanning correlation spectroscopy. Biophys J. 1990 Aug;58(2):503–511. doi: 10.1016/S0006-3495(90)82395-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. Microinjected carboxylated beads move predominantly poleward in sea urchin eggs. Cell Motil Cytoskeleton. 1987;8(4):293–301. doi: 10.1002/cm.970080402. [DOI] [PubMed] [Google Scholar]
- Weissman M., Schindler H., Feher G. Determination of molecular weights by fluctuation spectroscopy: application to DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2776–2780. doi: 10.1073/pnas.73.8.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]



