Abstract
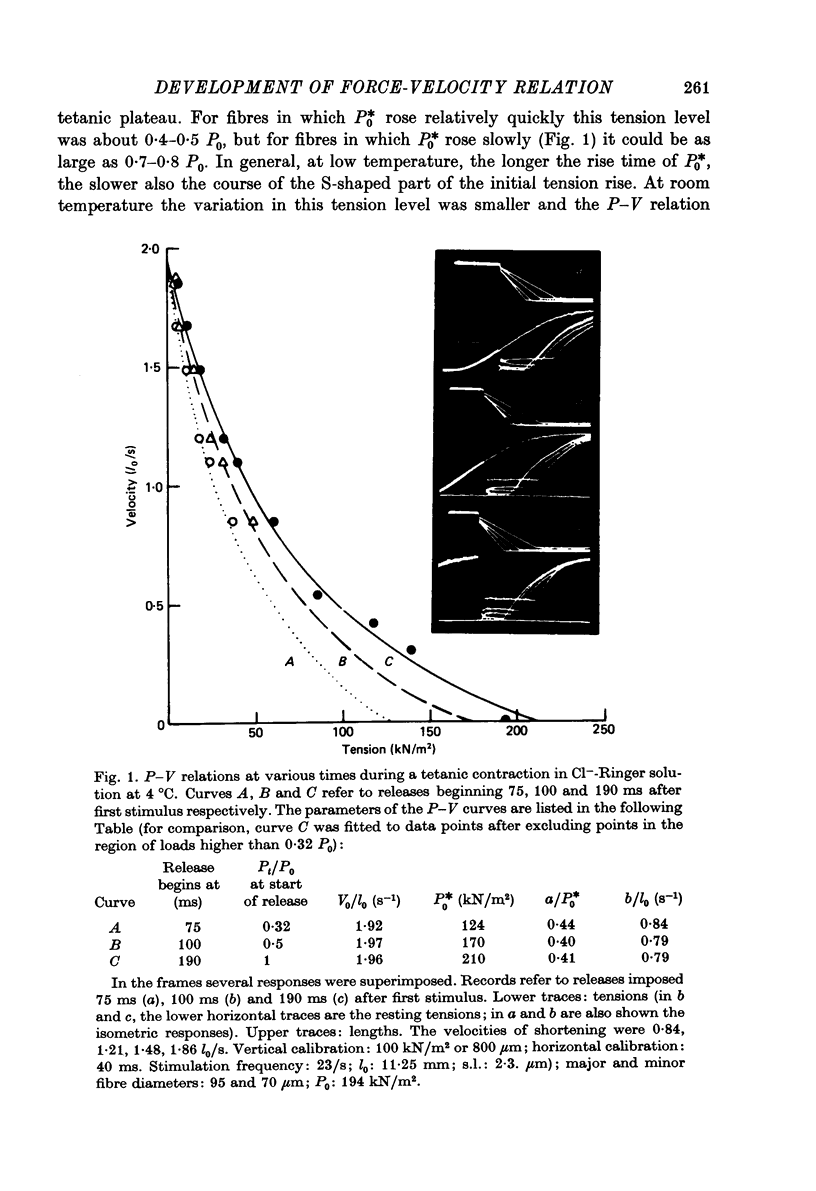
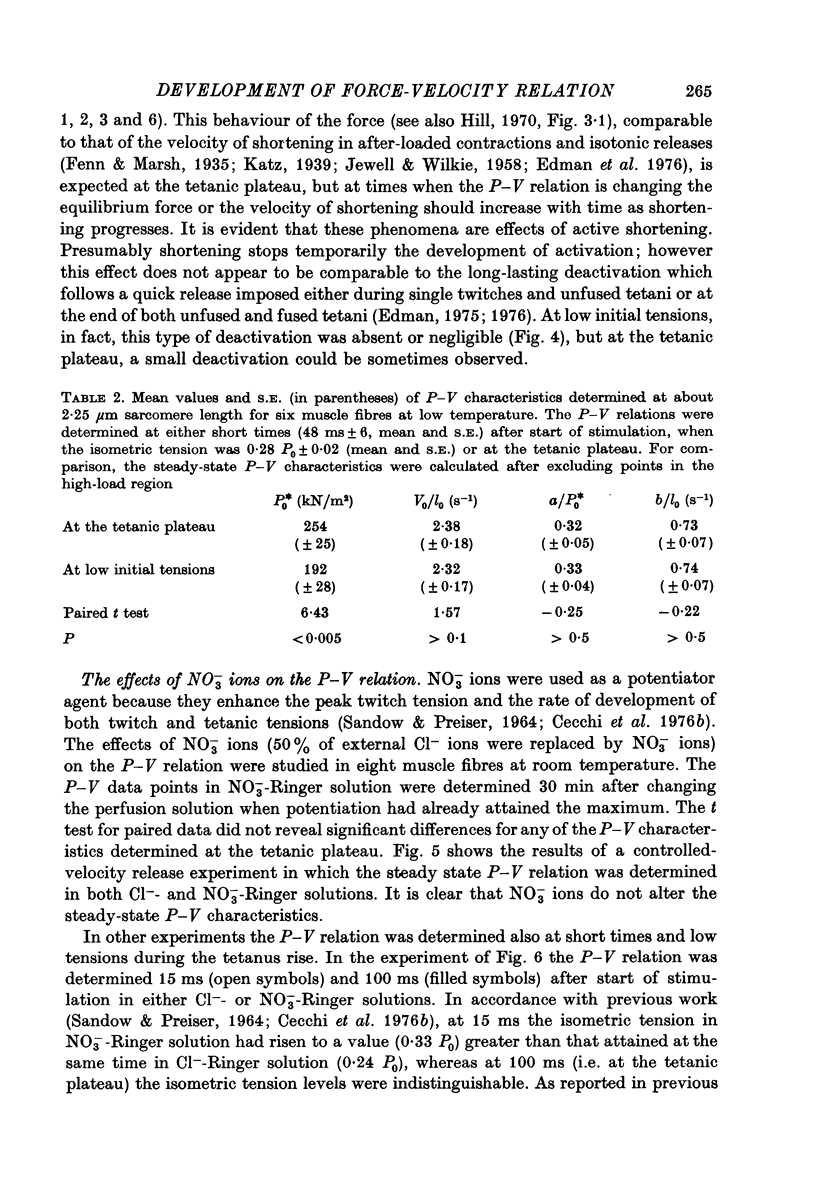
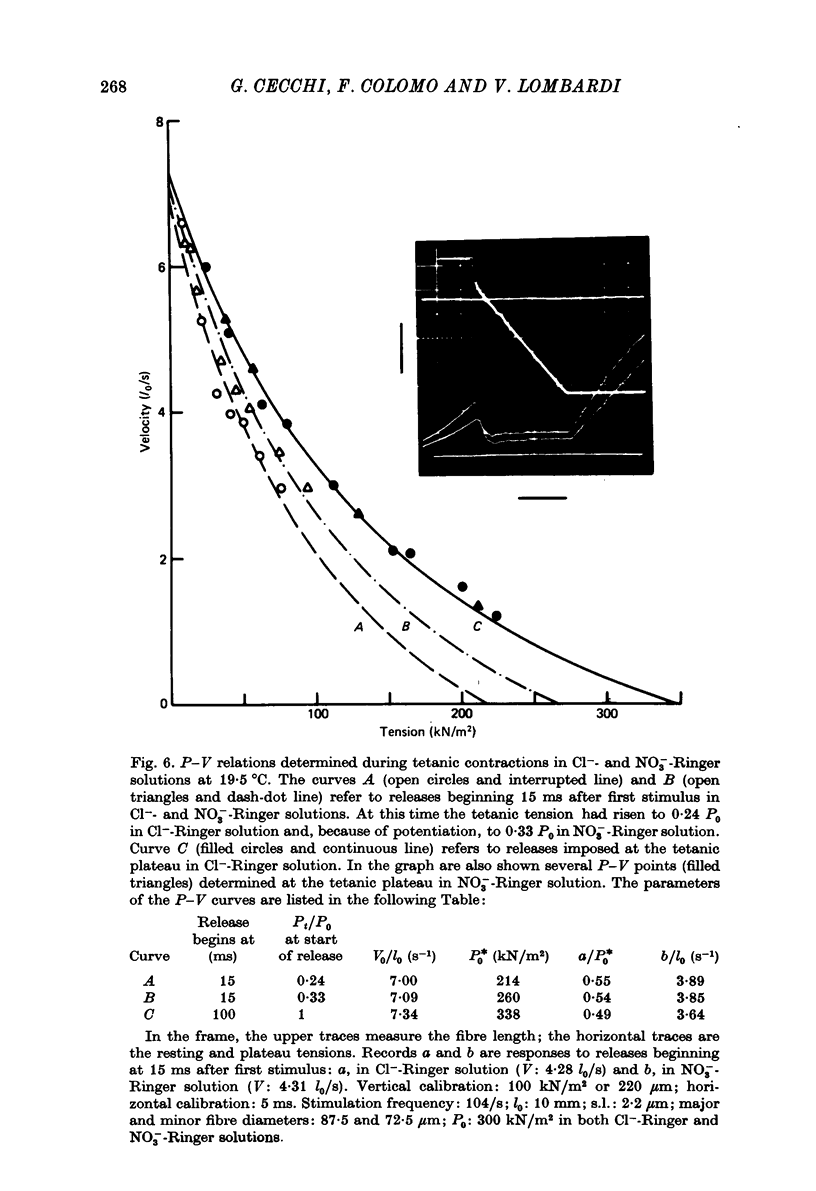
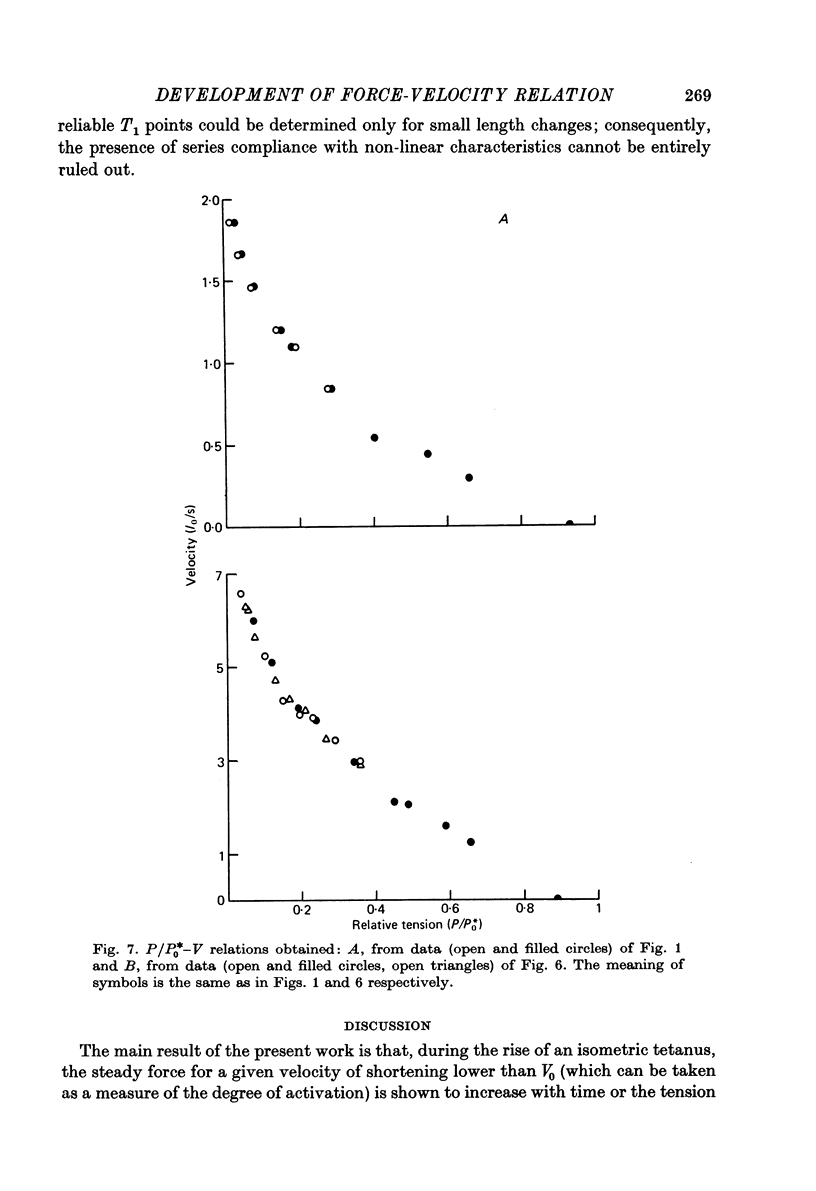
1. The force-velocity (P-V) relation for normal or NO-3 treated single fibres isolated from the semitendinosus muscle of the frog was determined at given times during the rise of tension and the plateau of isometric tetani. Experiments were made at about 2.25 micron sarcomere length and at constant temperatures, from 3 to 4.5 degrees C and from 19 to 21 degrees C. The controlled-velocity release method was used. 2. During the rise of tension, at any initial tension higher than about 0.2 P0, the lowest release velocity required to drop the tension to zero was the same as at the tetanic plateau, independent of the temperature and the presence of NO-3 ions in the bathing solution. 3. The degree of activation (measured by the steady force exerted at a given velocity of shortening lower than V0) increased with time, but attained its steady-state level before isometric tension. 4. At about 20 degrees C, frog muscle fibres at about 2.2 micron sarcomere length were only partially activated after a single stimulus. 5. NO-3 ions did not affect the steady-state P-V relation. At about 20 degrees C, NO-3 ions increased the rate of development of activation. Potentiation of the twitch contraction was due at least in part to this mechanism. 6. The 'relative' P-V relation appears to be independent of both the time after start of stimulation and the presence of NO-3 ions in the bathing solution. 7. The results are discussed in terms of the sliding filament model of Huxley (1957), assuming that either the number of actin sites available for cross-bridge formation, or the value of the rate constant for making of cross-bridges, is time dependent.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., RITCHIE J. M. The onset of shortening in striated muscle. J Physiol. 1951 Apr;113(2-3):336–345. doi: 10.1113/jphysiol.1951.sp004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G., Rose R. M. Proceedings: Aequorin-light and tension responses from bundles of myofibrils following a sudden change in free calcium. J Physiol. 1974 Sep;241(2):104P–106P. [PubMed] [Google Scholar]
- Bressler B. H., Clinch N. F. The compliance of contracting skeletal muscle. J Physiol. 1974 Mar;237(3):477–493. doi: 10.1113/jphysiol.1974.sp010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASELLA C. Tensile force in total striated muscle, isolated fibre and sarcolemma. Acta Physiol Scand. 1950 Dec;21(4):380–401. doi: 10.1111/j.1748-1716.1950.tb00744.x. [DOI] [PubMed] [Google Scholar]
- CLOSE R. The pattern of activation in the sartorius muscle of the frog. J Gen Physiol. 1962 Sep;46:1–18. doi: 10.1085/jgp.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. A loudspeaker servo system for determination of mechanical characteristics of isolated muscle fibres. Boll Soc Ital Biol Sper. 1976 May 30;52(10):733–736. [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. The variation of maximal tetanic tension during activity-dependent depression and potentiation of twitch contraction. Boll Soc Ital Biol Sper. 1976 May 30;52(10):723–725. [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. Two phases of NO-3-potentiation and the effects of this ion on the sarcomere length -twitch tension -tetanic tension curves in isolated muscle fibres of the frog. Boll Soc Ital Biol Sper. 1976 May 30;52(10):726–732. [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V. [The relation between the sarcomere length and the isometric twitch tension in isolated frog muscle fibres at 20 degrees C]. Boll Soc Ital Biol Sper. 1976 May 30;52(10):719–722. [PubMed] [Google Scholar]
- Close R. I. The relations between sarcomere length and characteristics of isometric twitch contractions of frog sartorius muscle. J Physiol. 1972 Feb;220(3):745–762. doi: 10.1113/jphysiol.1972.sp009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R., Hoh J. F. The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol. 1968 Jul;197(2):461–477. doi: 10.1113/jphysiol.1968.sp008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature. 1968 Feb 10;217(5128):529–532. doi: 10.1038/217529a0. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Depression of mechanical activity induced by active shortening in frog skeletal muscle fibres. Acta Physiol Scand. 1976 Nov;98(3):384–386. doi: 10.1111/j.1748-1716.1976.tb10325.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The rising phase of the active state in single skeletal muscle fibres of the frog. Acta Physiol Scand. 1970 Jun;79(2):167–173. doi: 10.1111/j.1748-1716.1970.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Marsh B. S. Muscular force at different speeds of shortening. J Physiol. 1935 Nov 22;85(3):277–297. doi: 10.1113/jphysiol.1935.sp003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., MACPHERSON L. The effect of nitrate, iodide and bromide on the duration of the active state in skeletal muscle. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):81–102. doi: 10.1098/rspb.1954.0055. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Mechanical properties of the cross-bridges of frog striated muscle. J Physiol. 1971 Oct;218 (Suppl):59P–60P. [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. D., Loomis T. A. Changes in velocity of muscle shortening during post-tetanic potentiation and during PMA induced twitch potentiation of the rat anterior tibial muscle. Life Sci. 1965 Dec;4(23):2309–2314. doi: 10.1016/0024-3205(65)90254-7. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., WILKIE D. R. The dynamics of muscular contraction. J Physiol. 1958 Aug 29;143(1):104–113. doi: 10.1113/jphysiol.1958.sp006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOW A., PREISER H. MUSCULAR CONTRACTION AS REGULATED BY THE ACTION POTENTIAL. Science. 1964 Dec 11;146(3650):1470–1472. doi: 10.1126/science.146.3650.1470. [DOI] [PubMed] [Google Scholar]
- SANDOW A., SEAMAN T. MUSCLE SHORTENING VELOCITY IN NORMAL AND POTENTIATED CONTRACTIONS. Life Sci. 1964 Feb;3:91–96. doi: 10.1016/0024-3205(64)90185-7. [DOI] [PubMed] [Google Scholar]


