Abstract
Infection with cag+ but not cag-negative Helicobacter pylori leads to the formation of large homotypic aggregates of macrophage-like cells. Intracellular adhesion molecule 1 is up-regulated and recruited to the cell surface of infected cells and mediates the aggregation via lymphocyte function-associated molecule 1. This signaling may regulate cell-cell interactions and inflammatory responses.
Helicobacter pylori is considered to play a major role in the development of gastric disease, such as chronic gastritis, peptic ulceration, or even cancer (6, 20). The mechanism of H. pylori pathogenesis is not well understood. This bacterium induces several host responses in epithelial cells, such as cell vacuolation (8), actin rearrangements resulting in a hummingbird or “scattering” phenotype (2, 13, 25, 27) and the secretion of a large set of inflammatory molecules such as interleukin-1 (IL-1), IL-6, tumor necrosis factor α, IL-8, Gro-α, RANTES, ENA-78, MCP-1, and Mip-1α (4, 18). One of these factors, namely IL-8, is thought to play a key role in the H. pylori infection process (5, 7), because it leads to a dense infiltration of different monocytic cells resulting in acute inflammation of the gastric mucosa (4). However, despite a strong inflammatory response, bacteria survive in the gastric mucosa for years or even decades without being cleared by the immune system of the host.
Several observations were made when the interaction of H. pylori with monocytic cells was studied. After infection of cultured J774.A cells, bacteria undergo delayed phagocytosis and megasomes form as described previously (1). During infection of monocytic cells derived from human blood, antiphagocytosis of H. pylori was observed (24). It has been demonstrated that these effects depended on a functional type IV secretion system encoded in the cag (cytotoxin-associated genes) pathogenicity island (PAI) of H. pylori. In an earlier study, we and others demonstrated that once cag+ H. pylori cells are attached to monocytic cells (J774.A, THP-1, U937, or Josk-M), the only known substrate of the type IV secretion system, the CagA protein is translocated into the host cells, followed by tyrosine phosphorylation and processing events (19, 22). The role of CagA in monocytic cells is still unknown. Here, we describe a new phenotype associated with H. pylori infections of macrophage-like cells.
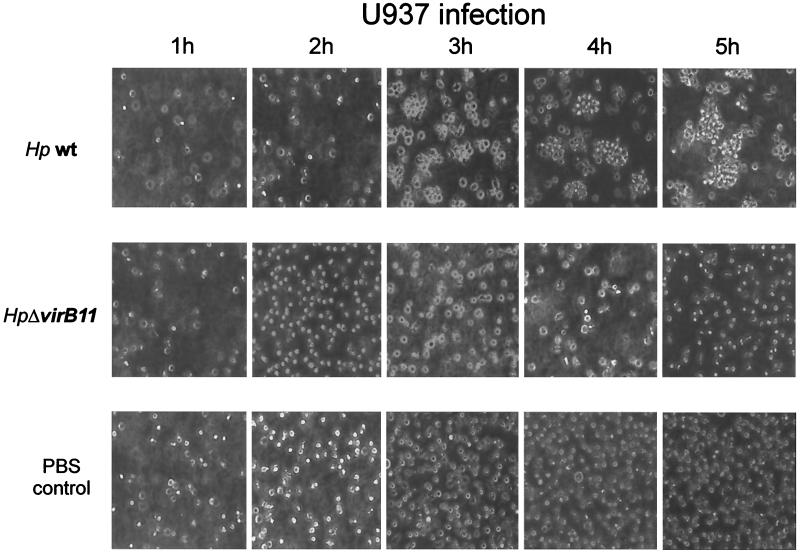
We differentiated U937 and Josk-M into macrophage-like cells and infected them with several cag+ H. pylori strains by using a multiplicity of infection of 100, as described previously (19). Differentiated U937 and Josk-M cells became partially adherent and were able to phagocytose opsonized Zymosan particles (Sigma). In a first experiment, we investigated the infected cells by light microscopy and determined the levels of IL-8 secreted into the culture medium. Infected cells formed aggregates consisting of two to five cells after 60 min (Fig. 1). After 3 h of infection, larger aggregates (>10 infected cells) occurred, and the phenotype was fully established after 5 h (Fig. 1). The aggregates remained stable for 24 h and did not disassemble over time. This homotypic aggregation of U937 and Josk-M cells was observed after infection with different cag+ H. pylori strains (Table 1). In contrast, U937 cells infected with H. pylori lacking the entire PAI (P12ΔcagPAI) did not form aggregates and resembled noninfected control cells, implying that virulence (vir) genes encoded in this PAI were necessary for the induction of the cellular phenotype. To further investigate the role of the cagPAI genes for the phenotypical outcome, we infected U937 and Josk-M cells with different H. pylori vir gene insertion knockout mutants (2). All isogenic virB-knockout mutants (ΔvirB4, ΔvirB7, ΔvirB8, ΔvirB9, ΔvirB10, and ΔvirB11) of the type IV secretion system failed to induce aggregation (summarized in Table 1). Surprisingly, infection of these macrophage-like cells with an H. pylori P1ΔcagA mutant led to the aggregation as observed for wild-type bacteria. We also determined IL-8 levels in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) after 8 h of infection by using the CytoSets system (BioSource International, Camarillo, Calif.). As expected, the highest levels of IL-8 were detected for cag+ H. pylori-infected cells, whereas infection with all isogenic virB-knockout mutants of the type IV secretion system did not induce IL-8 in the supernatant above background levels (Table 1). U937 and Josk-M cells infected with the H. pylori cagA mutant secreted nearly as much IL-8 as cag+ bacterium-infected cells (Table 1). This indicated that the homotypic aggregation parallels the induction of IL-8 proceeding in both a cagPAI-dependent manner and a CagA-independent manner.
FIG. 1.
Homotypic cell aggregation of macrophage-like cells induced by cag+ H. pylori. Photomicrographs (×20) of differentiated U937 (106 cells) cells that were infected with the H. pylori P1 wild type (upper panel) or P1ΔvirB mutant (middle panel) over a time course. Addition of PBS served as a control (lower panel). Results are representative of at least five independent experiments.
TABLE 1.
Induction of IL-8 and homotypic aggregation in U937 and Josk-M macrophage-like cells infected with H. pyloria
| H. pylori strain | Mutated gene | Open reading frameb | IL-8 secretionc | Homotypic aggregation phenotype |
|---|---|---|---|---|
| P1 wt | +++ | +++ | ||
| G27 wt | +++ | +++ | ||
| P12 wt | +++ | +++ | ||
| P12 | cagPAI | Hp0520-Hp0547 | − | − |
| P1 | virB4 | Hp0544 | − | − |
| P1 | virB7 | Hp0532 | − | − |
| P1 | virB8 | Hp0529 | − | − |
| P1 | virB9 | Hp0528 | − | − |
| P1 | virB10 | Hp0527 | − | − |
| P1 | virB11 | Hp0525 | − | − |
| P1 | cagA | Hp0547 | +++ | ++ |
All wild-type (wt) H. pylori strains are cag+ isolates. All mutants were created by insertion of chloramphenicol or kanamycin resistance gene cassettes, respectively, and were described previously (19, 26). Results are representative of at least three independent experiments.
Open reading frame nomenclature according to The Institute for Genomic Research sequence for H. pylori strain 26695 (http://www.tigr.org/tdb/).
IL-8 levels in the supernatant were determined by ELISA. The level of IL-8 in the supernatant (+++) is equivalent to 500 to 800 μg/ml. −, ≤50 μg/ml.
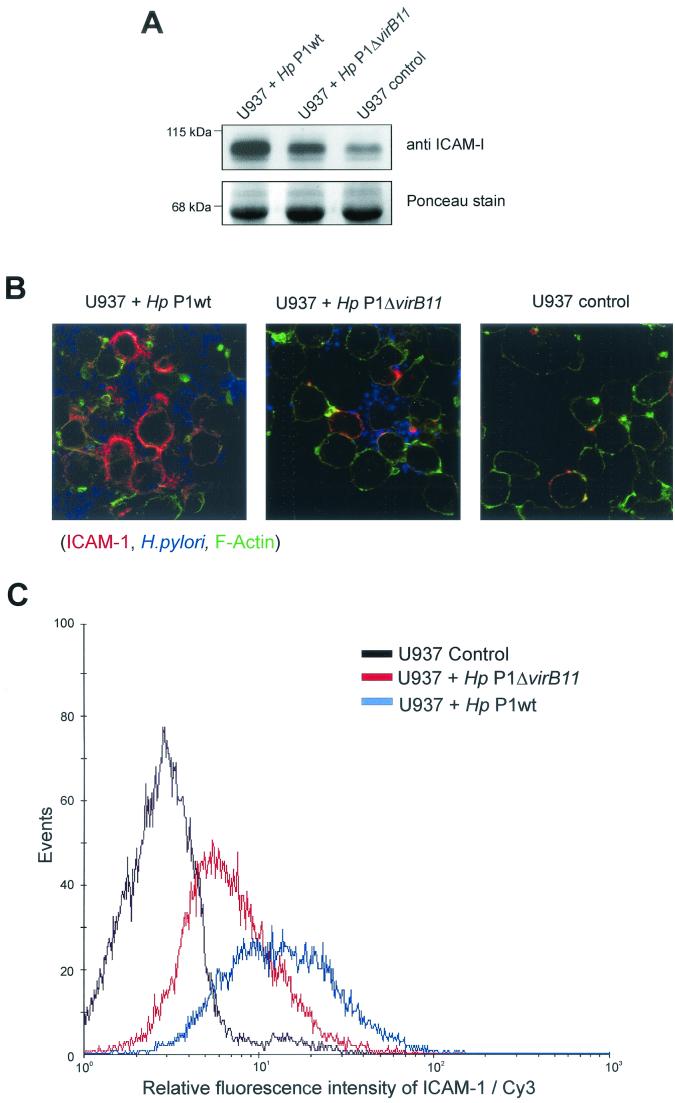
Homotypic aggregation has been associated with activation of B cells, T cells, monocytes, and neutrophils, but the molecular mechanism is widely unknown (3, 15). Investigation of biopsy samples from patients infected with H. pylori had previously shown that intracellular adhesion molecule 1 (ICAM-1) is the prominent adhesion molecule up-regulated in inflammatory cells (12, 14). In order to determine whether ICAM-1 may play a role in the induction of the homotypic aggregation phenotype in our experiments, we analyzed the expression of ICAM-1 during infection of U937 and Josk-M cells. For this purpose, Western blots were probed with an anti-ICAM-1 antibody (BD Transduction C10020; mouse monoclonal antibody) and an antimouse secondary antibody (Amersham antimouse-horseradish peroxidase conjugate) (Fig. 2 A). The highest level of ICAM-1 was detected for cag+ H. pylori-infected cells. Less ICAM-1 was detected in virB mutant-infected cells, and noninfected U937 control cells expressed low levels of ICAM-1 (Fig. 2A, upper panel). As a loading control, the blot was stained with Ponceau S red (Sigma) (Fig. 2A, lower panel).
FIG. 2.
Up-regulation of the cellular adhesion molecule ICAM-1 after H. pylori infection. Differentiated U937 cells were infected for 6 h with cag+ H. pylori, an isogenic H. pylori knockout strain (ΔvirB11), or PBS as a control. (A) ICAM-1 expression of whole-cell lysates of infected U937 cells. Proteins from infected cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and ICAM-1 expression was investigated by immunoblotting with anti-ICAM-1 antibody (upper panel). Staining of the blot with Ponceau S red was used as a loading control (lower panel). (B) Confocal microscopy analysis of infected macrophage-like cells. U937 cells were infected with H. pylori for 6 h, fixed and stained with anti-ICAM-1-Cy3 (red), anti-H. pylori-Cy5 (blue), and phalloidin-Alexa 488 (green). (C) FACS analysis of ICAM-1 surface expression. U937 cells were fixed after infection and stained with anti-ICAM-1-Cy3. Results were confirmed in three independent experiments.
To directly visualize ICAM-1 in infected cells, confocal microscopy was applied. For this purpose, U937 cells were stained with an anti-ICAM-1 mouse antibody (DAKO ICAM-1/CD54), an anti-H. pylori rabbit antibody (Biomol), and phalloidin-Alexa 488 conjugate (A-12379; Molecular Probes) according to standard procedures (Santa Cruz). Fluorescence labeling was performed with an antimouse Cy3 conjugate (no. 115-165-146; Jackson ImmunoResearch Laboratories) and an antirabbit Cy5 conjugate (111-175-144; Jackson ImmunoResearch Laboratories). ICAM-1 accumulated at the membrane of cells infected with cag+ H. pylori (Fig. 2B, left panel). In contrast, all virB mutants induced substantially less ICAM-1 on the surface of the infected cells (Fig. 2B, middle panel), while in the noninfected control, only single cells expressing ICAM-1 were detected (Fig. 2B, right panel).
Surface expression of ICAM-1 was analyzed by cytofluorometry. After 6 h of infection, U937 cells (106 cells) were fixed in 4% paraformaldehyde for 20 min and washed three times with fluorescence-activated cell sorting (FACS) buffer (0.5% bovine serum albumin [BSA] in phosphate-buffered saline [PBS]). The fixed cells were incubated with an anti-ICAM-1 antibody (DAKO CD54 mouse monoclonal antibody) for 20 min at 4°C and after three additional washing steps (FACS buffer) incubated with anti-mouse Cy3 secondary antibody for 20 min at 4°C. Subsequent FACS analysis showed two different effects that occur after H. pylori infection. First, a general up-regulation of ICAM-1 at the cell surface following virB mutant infection can be observed, as indicated by the fluorescence shift (mean, 6.65; median, 6). Second, an increased fluorescence shift (mean, 13.13; median, 13) for cag+-infected cells was obtained, suggesting additional induction of ICAM-1 at the cell surface. Noninfected control cells showed only background levels of ICAM-1 (mean, 2.67; median, 3). This suggested that massive recruitment of ICAM-1 to the cell surface by cag+ bacteria may lead to the homotypic aggregation of the cells.
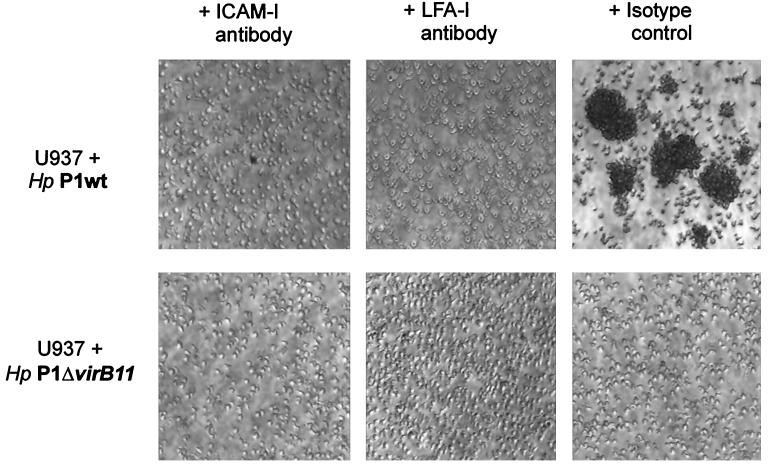
In order to evaluate whether the intercellular interaction of the infected cells could be mediated via a natural ligand of ICAM-1, lymphocyte function-associated molecule 1 (LFA-1), we performed blocking experiments with anti-ICAM-1 and anti-LFA-1 antibodies. Addition of the anti-ICAM-1 antibody (25 μg of antibody per 5 × 105 cells/ml) to the culture medium of U937 cells 30 min prior to infection completely abolished homotypic aggregation by cag+ H. pylori-infected cells (Fig. 3, left panel). The same results were obtained for blocking experiments with infected cells treated with an anti-LFA-1 antibody (CD11a monoclonal antibody mouse serum, no. 0157; Immunotech) (Fig. 3, middle panel). As a control, treatment of infected U937 cells with isotype control antibodies (Immunotech) did not affect aggregation (Fig. 3, right panel).
FIG. 3.
Blocking of homotypic aggregation by anti-ICAM-1 or LFA-1 antibodies. Photomicrographs (×20) of homotypic cell aggregation induced by cag+ H. pylori infection. Differentiated U937 (106 cells) were treated with anti-ICAM-1 antibody (25 μg of antibody per 5 × 105 cells) (A) or an equal amount of isotype control antibody (B) for 30 min prior to infection. Photomicrographs were taken 6 h after infection. Results are representative of at least three independent experiments.
In summary, H. pylori infection of U937 or Josk-M macrophage-like cells led to homotypic aggregation of the host cells. This was dependent on the functional integrity of the bacterial type IV secretion system, because all virB-knockout strains did not induce this phenotype. Immunofluorescence and FACS analysis further demonstrated that the adhesion molecule ICAM-1 is recruited to the host cell surface. Blocking experiments with anti-ICAM-1 and anti-LFA-1 antibodies revealed that the homotypic aggregation is mediated via ICAM-1 and LFA-1. Interestingly, the highest IL-8 levels in the supernatant of infected cells could be determined for cells infected with cag+ bacteria, suggesting that the observed phenotype correlates with the induction of IL-8 in these cells. Several groups demonstrated that the induction of proinflammatory cytokines like IL-8 correlates with the expression of different adhesion molecules, such as ICAM-1 (9, 21). For epithelial cells, Mori et al. (21) reported that ICAM-1 can be induced by cag+ H. pylori via activation of the transcription factor NF-κB. This led us to suggest that expression of both IL-8 and ICAM-1 may be induced in U937 and Josk-M cells via NF-κB. Activation of IL-8 and NF-κB in both epithelial cells (10, 11, 16, 17, 28) and macrophage-like cells (this study) is independent of CagA translocation. This supports the view that induction of the homotypic aggregation phenotype is triggered either (i) by translocation of a yet unknown bacterial effector molecule or (ii) by activation of host cell surface receptors induced by binding of the type IV secretion system itself. Alternatively, secreted IL-8 could activate the cells and thereby stimulate the ICAM-1 expression, but this is rather unlikely, because aggregation had already occurred 60 min after bacterial attachment. However, ICAM-1 expression is dramatically increased at the sites of gastric inflammation in vivo (12, 14, 23), thus providing important means of regulating cell-cell interactions and thereby presumably inflammatory responses. cag+ H. pylori-induced up-regulation of ICAM-1 and cytokines could contribute to or even enhance inflammatory cell recruitment and retention of these cells at the sites of infection. The role of the resulting phenotype in vivo (e.g., for the modulation of phagocytosis and other events) is under investigation.
Acknowledgments
We are grateful to Tasso Tsirpouchtsidis, Agnes Szczepek, and Toni Aebischer for critical discussion of the data.
This work was supported by a grant from the Fonds der Chemischen Industrie to T.F.M.
Editor: E. I. Tuomanen
REFERENCES
- 1.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 3.Bjorck, P., C. Elenstrom-Magnusson, A. Rosen, E. Severinson, and S. Paulie. 1993. CD23 and CD21 function as adhesion molecules in homotypic aggregation of human B lymphocytes. Eur. J. Immunol. 23:1771-1775. [DOI] [PubMed] [Google Scholar]
- 4.Bodger, K., and J. E. Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 54:139-150. [DOI] [PubMed] [Google Scholar]
- 5.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover, T. L., and H. J. Blaser. 1999. Helicobacter pylori factors associated with disease. Gastroenterology 117:257-261. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree, J. E., D. Kersulyte, S. D. Li, I. J. Lindley, and D. E. Berg. 1999. Modulation of Helicobacter pylori induced interleukin-8 synthesis in gastric epithelial cells mediated by cag PAI encoded VirD4 homologue. J. Clin. Pathol. 52:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bernard, M., M. Moschioni, G. Napolitani, R. Rappuoli, and C. Montecucco. 2000. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 19:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan, X. G., X. J. Fan, H. X. Xia, P. W. Keeling, and D. Kelleher. 1995. Up-regulation of CD44 and ICAM-1 expression on gastric epithelial cells by H. pylori. APMIS 103:744-748. [DOI] [PubMed] [Google Scholar]
- 10.Foryst-Ludwig, A., and M. Naumann. 2000. p21-activated kinase 1 activates the nuclear factorκB (NF-κB)-inducing kinase-IκB kinases NFκ-B pathway and proinflammatory cytokines in Helicobacter pylori infection. J. Biol. Chem. 275:39779-39785. [DOI] [PubMed] [Google Scholar]
- 11.Glocker, E., C. Lange, A. Covacci, S. Bereswill, M. Kist, and H. L. Pahl. 1998. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect. Immun. 66:2346-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatz, R. A., G. Rieder, M. Stolte, E. Bayerdorffer, G. Meimarakis, F. W. Schildberg, and G. Enders. 1997. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology 112:1908-1919. [DOI] [PubMed] [Google Scholar]
- 13.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, K., T. Arakawa, T. Uchida, K. Nakagawa, S. Nakamura, T. Matsumoto, T. Fukuda, K. Kobayashi, and T. Kuroki. 1997. In situ expression of cell adhesion molecules in chronic gastritis with Helicobacter pylori infection. J. Clin. Gastroenterol. 25:215-221. [DOI] [PubMed] [Google Scholar]
- 15.Isobe, K., and I. Nakashima. 1991. Homotypic aggregation of murine T lymphocytes induced by anti-Thy-1 monoclonal antibodies. Immunology 73:159-164. [PMC free article] [PubMed] [Google Scholar]
- 16.Isomoto, H., Y. Mizuta, M. Miyazaki, F. Takeshima, K. Omagari, K. Murase, T. Nishiyama, K. Inoue, I. Murata, and S. Kohno. 2000. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am. J. Gastroenterol. 95:2768-2776. [DOI] [PubMed] [Google Scholar]
- 17.Keates, S., Y. S. Hitti, M. Upton, and C. P. Kelly. 1997. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology 113:1099-1109. [DOI] [PubMed] [Google Scholar]
- 18.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, and A. M. Svennerholm. 2001. Induction of chemokine and cytokine responses by Helicobacter pylori in human stomach explants. Scand. J. Gastroenterol. 36:1022-1029. [DOI] [PubMed] [Google Scholar]
- 19.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 20.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 21.Mori, N., A. Ueda, R. Geleziunas, A. Wada, T. Hirayama, T. Yoshimura, and N. Yamamoto. 2001. Induction of monocyte chemoattractant protein 1 by Helicobacter pylori involves NF-κB. Infect. Immun. 69:1280-1286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 23.Ohara, T., T. Arakawa, K. Higuchi, and K. Kaneda. 2001. Overexpression of co-stimulatory molecules in peripheral mononuclear cells of Helicobacter pylori-positive peptic ulcer patients: possible difference in host responsiveness compared with non-ulcer patients. Eur. J. Gastroenterol. Hepatol. 13:11-18. [DOI] [PubMed] [Google Scholar]
- 24.Ramarao, N., S. D. Gray-Owen, S. Backert, and T. F. Meyer. 2000. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol. Microbiol. 37:1389-1404. [DOI] [PubMed] [Google Scholar]
- 25.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277:6775-6778. [DOI] [PubMed] [Google Scholar]
- 28.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]