Abstract
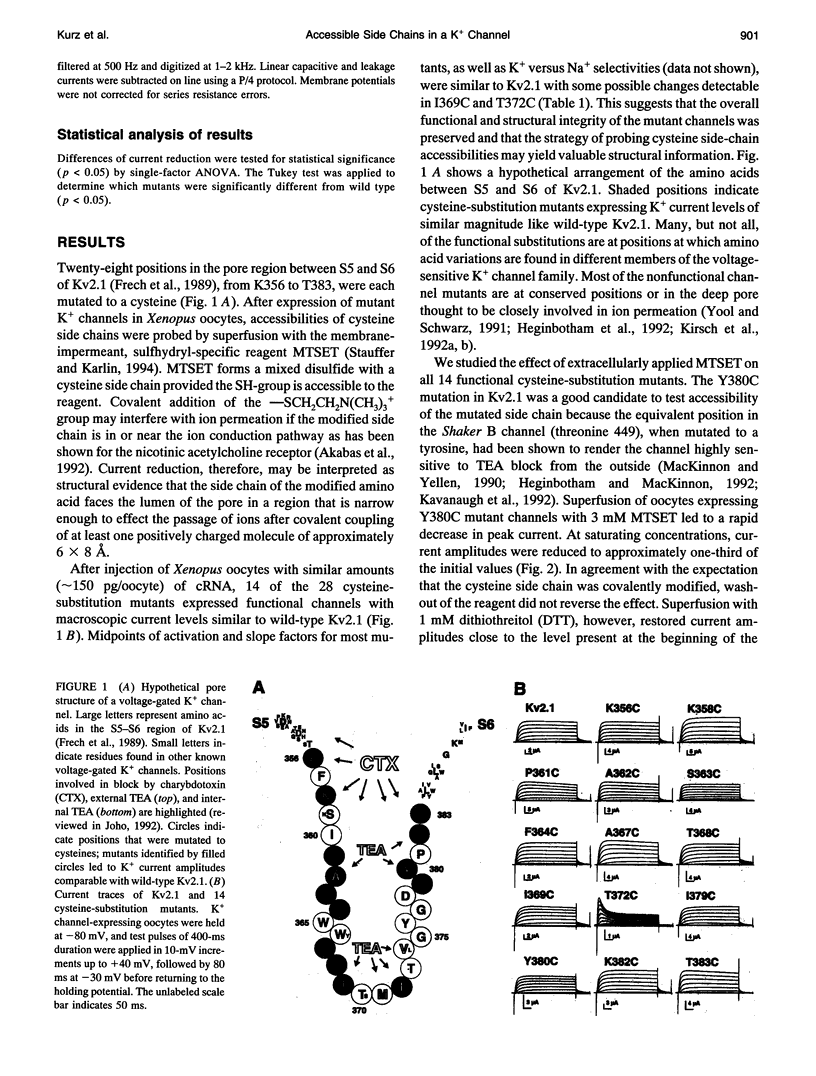
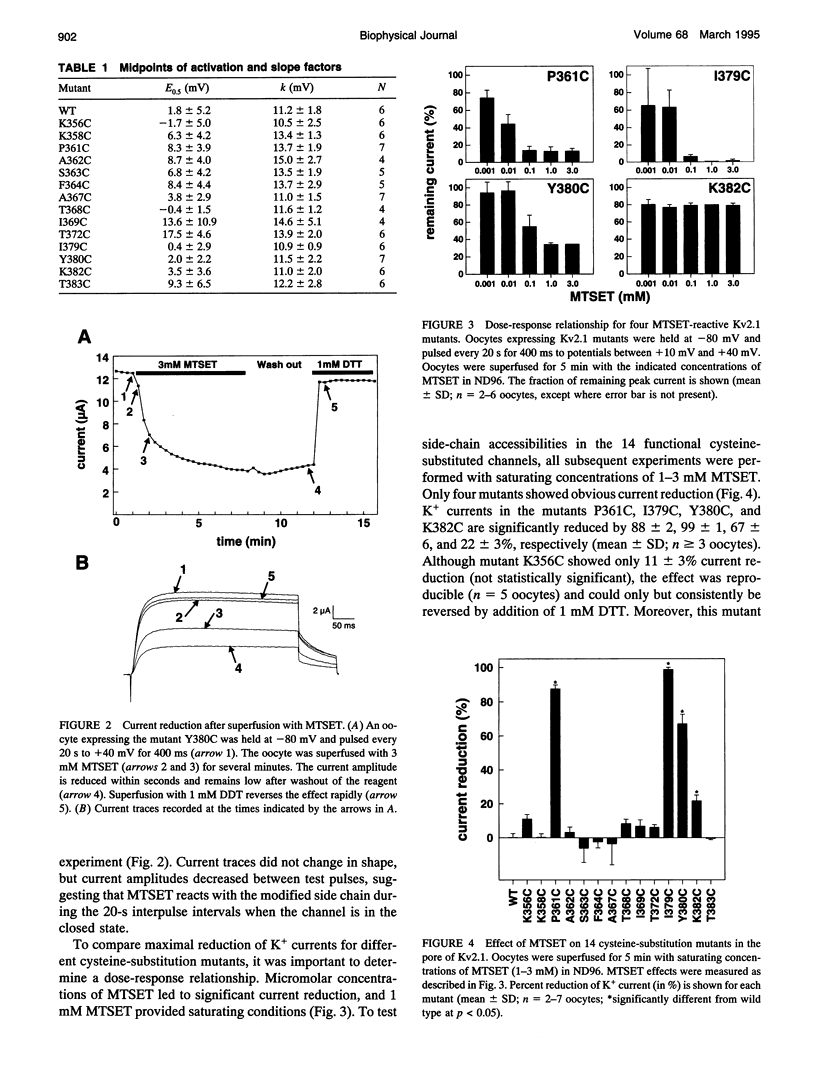
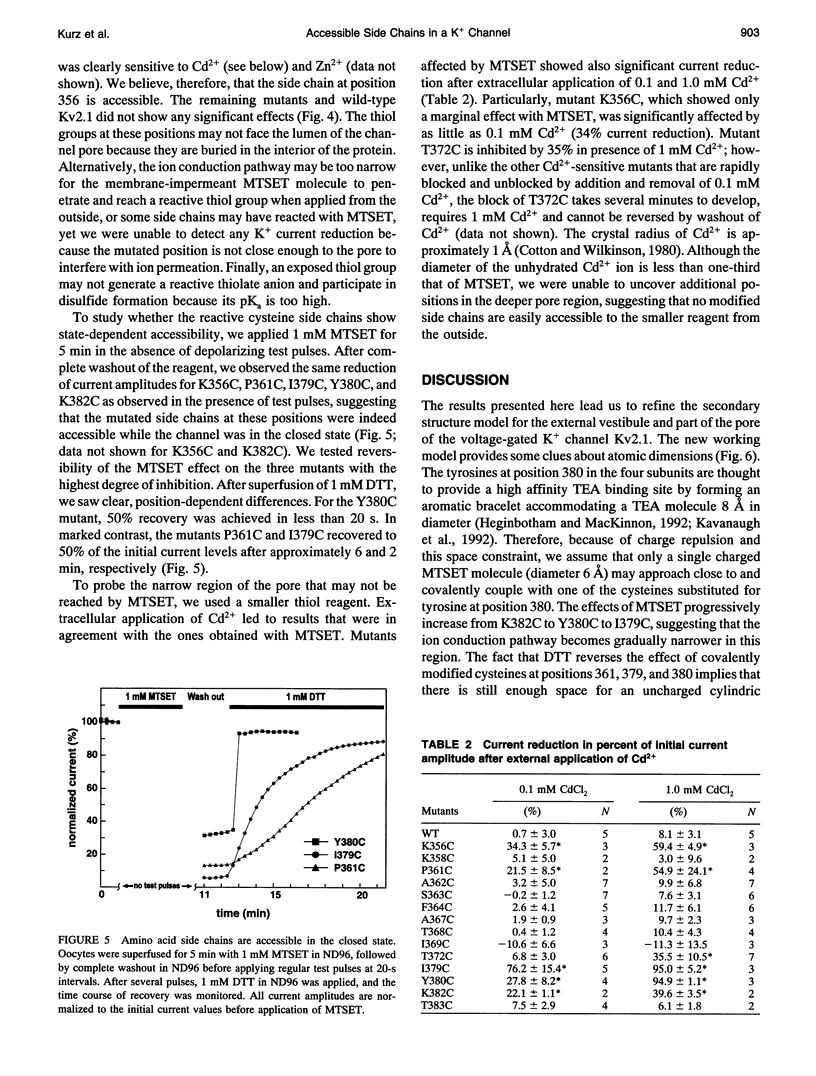
To gain insight into the secondary structure of the ion conduction pathway of a voltage-gated K+ channel, we used sulfhydryl-specific reagents of different diameters to probe amino acid side-chain accessibilities in the pore of the channel after cysteine-substitution mutagenesis. We identified five positions at which modified amino acid side chains are accessible from the aqueous lumen of the external channel vestibule. Covalent coupling of the 2-trimethylammonium-thioethyl group to cysteine thiols leads to position-dependent current reduction, suggesting a gradual narrowing of the pore. The fact that the modified side chains of two adjacent amino acids are reactive is not compatible with the ion conduction pathway forming a regular beta-pleated sheet at these positions. The smaller thiol reagent Cd2+ reacts with modified side chains that are also accessible to the larger (2-trimethylammoniumethyl)methanethiosulfate (MTSET) [corrected]. Our results imply that the outer vestibule of a potassium-selective ion channel narrows over a short distance of three amino acids near a position where a regular beta-structure is unlikely.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Bogusz S., Busath D. Is a beta-barrel model of the K+ channel energetically feasible? Biophys J. 1992 Apr;62(1):19–21. doi: 10.1016/S0006-3495(92)81765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated ion channels. Trends Neurosci. 1993 Dec;16(12):500–506. doi: 10.1016/0166-2236(93)90193-p. [DOI] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- De Biasi M., Drewe J. A., Kirsch G. E., Brown A. M. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+ pore. Biophys J. 1993 Sep;65(3):1235–1242. doi: 10.1016/S0006-3495(93)81154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Abramson T., MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992 Nov 13;258(5085):1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Tracing the roots of ion channels. Cell. 1992 May 29;69(5):715–718. doi: 10.1016/0092-8674(92)90280-p. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Hurst R. S., Yakel J., Varnum M. D., Adelman J. P., North R. A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992 Mar;8(3):493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Hartmann H. A., Taglialatela M., de Biasi M., Brown A. M., Joho R. H. Differences between the deep pores of K+ channels determined by an interacting pair of nonpolar amino acids. Neuron. 1992 Mar;8(3):499–505. doi: 10.1016/0896-6273(92)90278-l. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., DeBiasi M., Hartmann H. A., Brown A. M. A single nonpolar residue in the deep pore of related K+ channels acts as a K+:Rb+ conductance switch. Biophys J. 1992 Apr;62(1):136–144. doi: 10.1016/S0006-3495(92)81800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G. A., Jan Y. N., Jan L. Y. Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 1994 Jan 13;367(6459):179–182. doi: 10.1038/367179a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990 Oct 12;250(4978):276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Jan Y. N., Jan L. Y. The S4-S5 loop contributes to the ion-selective pore of potassium channels. Neuron. 1993 Oct;11(4):739–749. doi: 10.1016/0896-6273(93)90083-4. [DOI] [PubMed] [Google Scholar]
- Stauffer D. A., Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994 Jun 7;33(22):6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990 Oct;5(4):433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994 Apr;66(4):1068–1075. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Zühlke R. D., Zhang H. J., Joho R. H. Role of an invariant cysteine in gating and ion permeation of the voltage-sensitive K+ channel Kv2.1. Receptors Channels. 1994;2(3):237–248. [PubMed] [Google Scholar]