Abstract
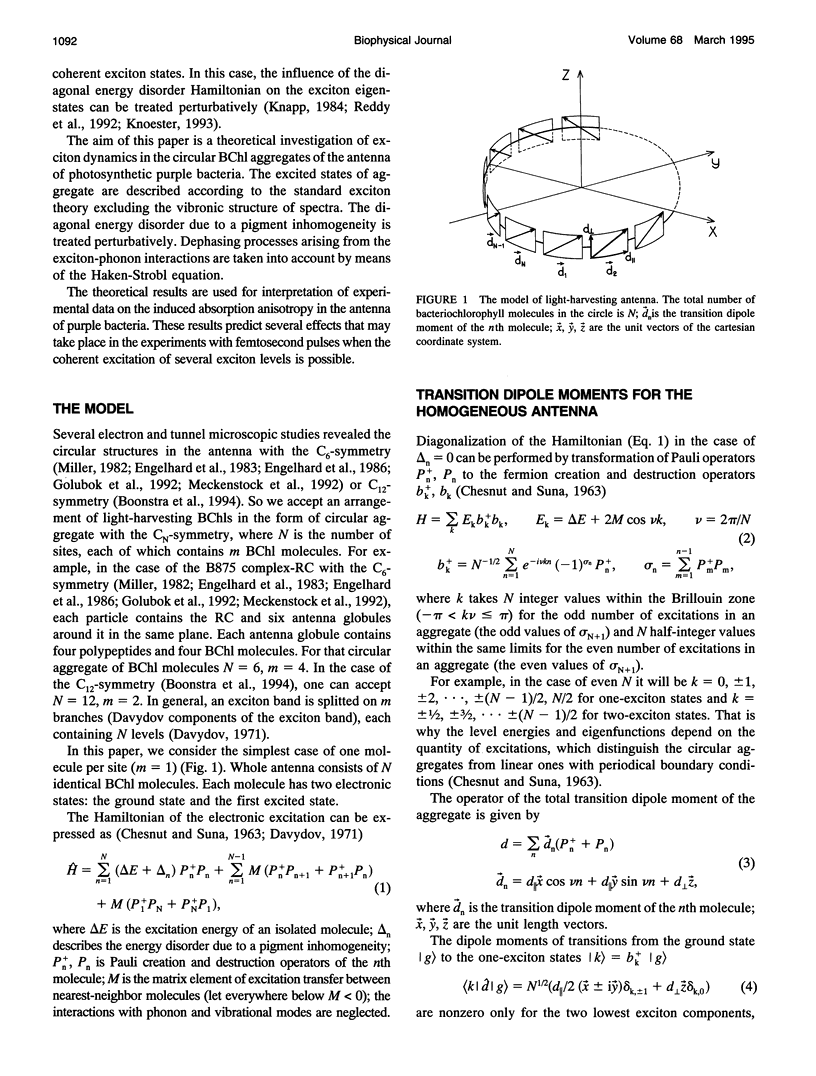
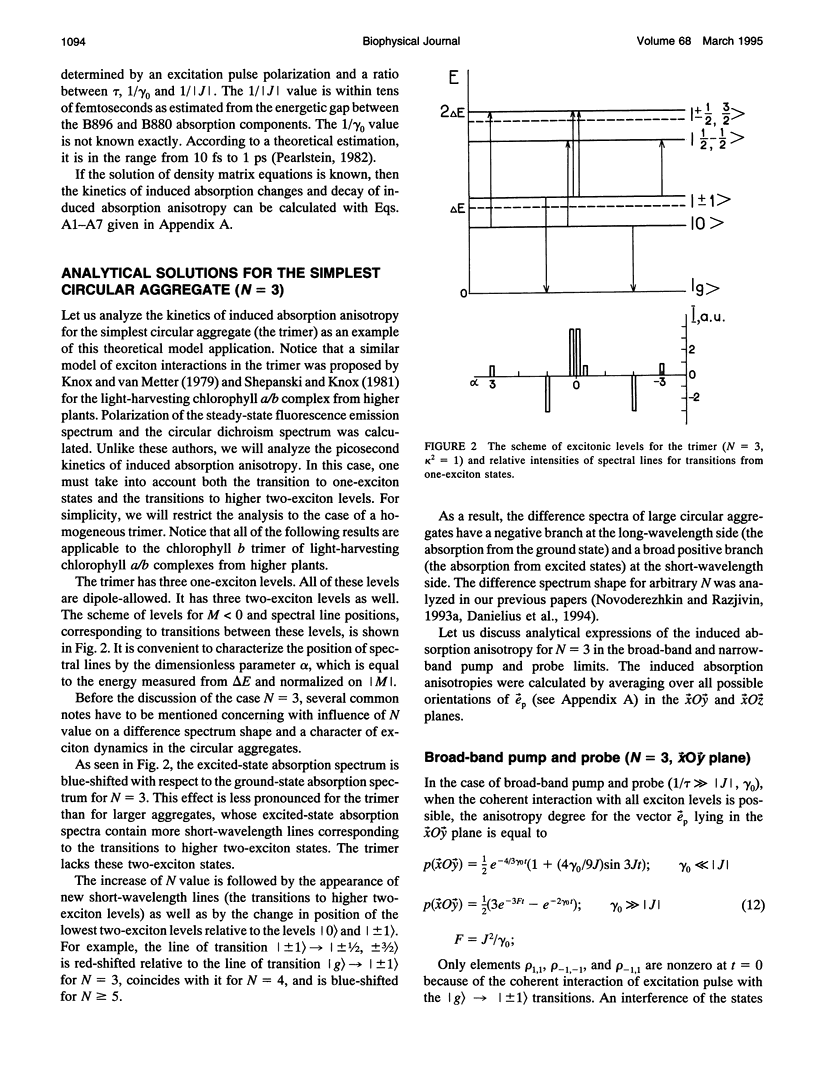
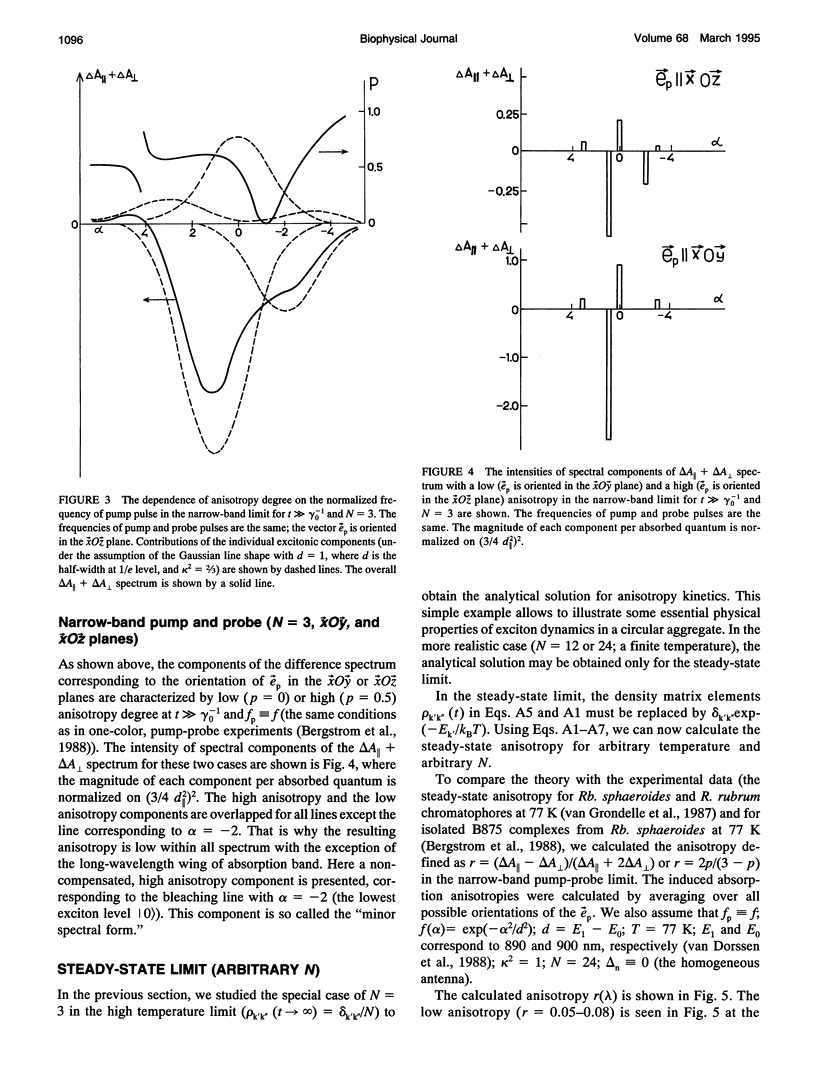
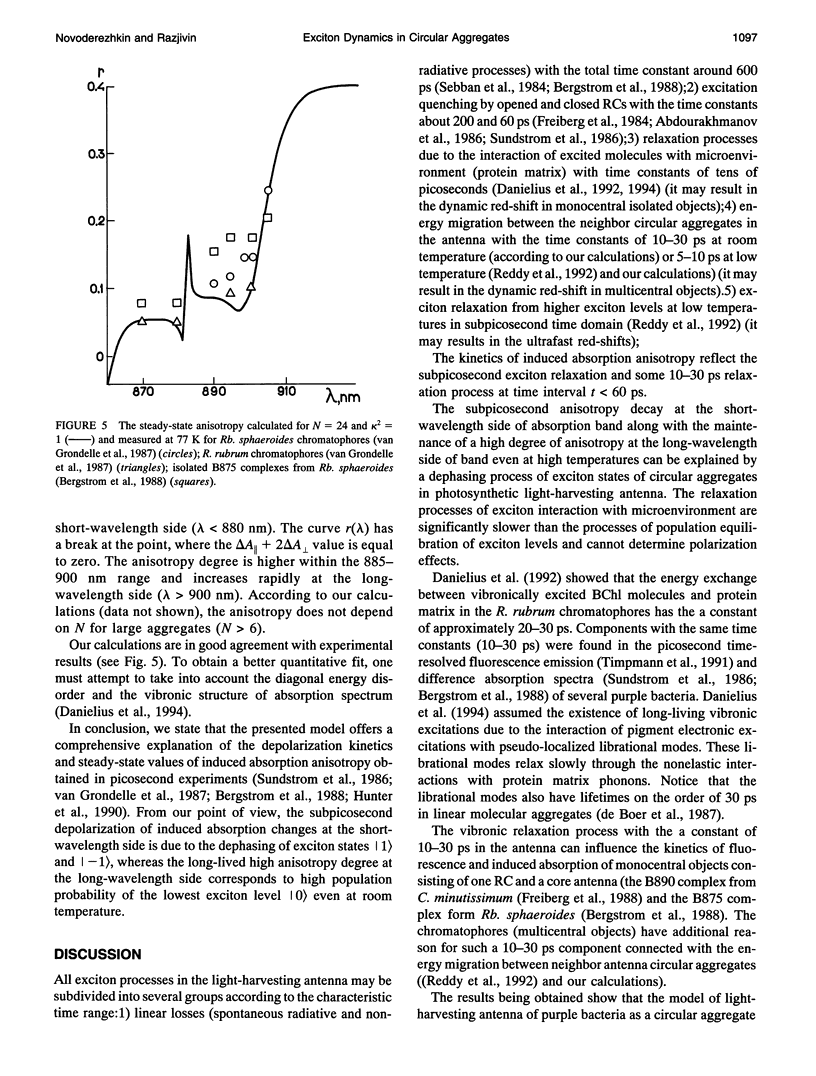
A theoretical model of exciton dynamics in circular molecular aggregates of light-harvesting bacteriochlorophyll of photosynthetic bacteria is proposed. The spectra and anisotropy of photoinduced absorption changes in the femto- and picosecond time domain are under its scope. The excited state of aggregate was treated due to the standard exciton theory, taking into account a pigment inhomogeneity. Dephasing processes via the exciton-phonon interactions were described by means of the Haken-Strobl equation. It was shown that only two exciton levels are dipole-allowed in the case of homogeneous circular aggregate. The pigment inhomogeneity results in the appearance of several weak transitions to higher exciton levels. It was proposed that the minor band (B896) in an absorption spectrum of the B875 complex as well as the similar minor band in spectra of B800-850 complex correspond to electron transition from the ground to the lowest exciton level, whereas the major band corresponds to transition to the higher exciton level. The proposed model shows the subpicosecond decay of anisotropy at the short-wavelength side of absorption band and a high degree of anisotropy at the long-wavelength side, even at high temperatures.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P., Scherz A. Polypeptides and bacteriochlorophyll organization in the light-harvesting complex B850 of Rhodobacter sphaeroides R-26.1. Biochemistry. 1991 May 28;30(21):5177–5184. doi: 10.1021/bi00235a010. [DOI] [PubMed] [Google Scholar]
- Danielius R., Novoderezhkin V., Razjivin A. Picosecond absorbance difference spectra of the antenna of photosynthetic purple bacteria. The influence of exciton interactions and librations. FEBS Lett. 1994 May 30;345(2-3):203–206. doi: 10.1016/0014-5793(94)00428-5. [DOI] [PubMed] [Google Scholar]
- Drews G. Structure and functional organization of light-harvesting complexes and photochemical reaction centers in membranes of phototrophic bacteria. Microbiol Rev. 1985 Mar;49(1):59–70. doi: 10.1128/mr.49.1.59-70.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H., Engel A., Baumeister W. Stoichiometric model of the photosynthetic unit of Ectothiorhodospira halochloris. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8972–8976. doi: 10.1073/pnas.83.23.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godik V., Timpmann K., Freiberg A., Moskalenko A. A. Picosecond dynamics of excitations in light-harvesting complex B800-850 from Chromatium minutissimum studied using fluorescence spectrochronography. FEBS Lett. 1993 Jul 19;327(1):68–70. doi: 10.1016/0014-5793(93)81041-w. [DOI] [PubMed] [Google Scholar]
- Grad J, Hernandez G, Mukamel S. Radiative decay and energy transfer in molecular aggregates: The role of intermolecular dephasing. Phys Rev A Gen Phys. 1988 May 15;37(10):3835–3846. doi: 10.1103/physreva.37.3835. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., Bergström H., van Grondelle R., Sundström V. Energy-transfer dynamics in three light-harvesting mutants of Rhodobacter sphaeroides: a picosecond spectroscopy study. Biochemistry. 1990 Apr 3;29(13):3203–3207. doi: 10.1021/bi00465a008. [DOI] [PubMed] [Google Scholar]
- Meckenstock R. U., Krusche K., Brunisholz R. A., Zuber H. The light-harvesting core-complex and the B820-subunit from Rhodopseudomonas marina. Part II. Electron microscopic characterisation. FEBS Lett. 1992 Oct 19;311(2):135–138. doi: 10.1016/0014-5793(92)81384-x. [DOI] [PubMed] [Google Scholar]
- Novoderezhkin V. I., Razjivin A. P. Excitonic interactions in the light-harvesting antenna of photosynthetic purple bacteria and their influence on picosecond absorbance difference spectra. FEBS Lett. 1993 Sep 6;330(1):5–7. doi: 10.1016/0014-5793(93)80907-c. [DOI] [PubMed] [Google Scholar]
- Pullerits T., Freiberg A. Kinetic model of primary energy transfer and trapping in photosynthetic membranes. Biophys J. 1992 Oct;63(4):879–896. doi: 10.1016/S0006-3495(92)81688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits T., Visscher K. J., Hess S., Sundström V., Freiberg A., Timpmann K., van Grondelle R. Energy transfer in the inhomogeneously broadened core antenna of purple bacteria: a simultaneous fit of low-intensity picosecond absorption and fluorescence kinetics. Biophys J. 1994 Jan;66(1):236–248. doi: 10.1016/S0006-3495(94)80770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen O. J., van Mourik F., van Grondelle R., Valkunas L. Energy migration and trapping in a spectrally and spatially inhomogeneous light-harvesting antenna. Biophys J. 1994 May;66(5):1580–1596. doi: 10.1016/S0006-3495(94)80950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano FC, Kuklinski JR, Mukamel S. Temperature-dependent superradiant decay of excitons in small aggregates. Phys Rev Lett. 1990 Jul 9;65(2):211–214. doi: 10.1103/PhysRevLett.65.211. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Cogdell R. J., Pierson B. K., Seftor R. E. Pigment-protein complexes of purple photosynthetic bacteria: an overview. J Cell Biochem. 1983;23(1-4):159–169. doi: 10.1002/jcb.240230113. [DOI] [PubMed] [Google Scholar]
- Van Metter R. L. Excitation energy transfer in the light-harvesting chlorophyll a/b.protein. Biochim Biophys Acta. 1977 Dec 23;462(3):642–658. doi: 10.1016/0005-2728(77)90107-4. [DOI] [PubMed] [Google Scholar]


