Abstract
The Helicobacter pylori vacuolating cytotoxin (VacA) induces the degenerative vacuolation of mammalian cells both in vitro and in vivo. Here, we demonstrate that plasma membrane cholesterol is essential for vacuolation of mammalian cells by VacA. Vacuole biogenesis in multiple cell lines was completely blocked when cholesterol was extracted selectively from the plasma membrane by using β-cyclodextrins. Moreover, increasing plasma membrane cholesterol levels strongly potentiated VacA-induced vacuolation. In contrast, inhibiting de novo biosynthesis of cholesterol with lovastatin or compactin had no detectable effect on vacuolation. While depletion of plasma membrane cholesterol has been shown to interfere with both clathrin-mediated endocytosis and caveola-dependent endocytosis, neither of these two internalization pathways was found to be essential for vacuolation of cells by VacA. Depleting plasma membrane cholesterol attenuated the entry of VacA into HeLa cells. In addition, β-cyclodextrin reagents blocked vacuolation of cells that were either preloaded with VacA or had VacA directly expressed within the cytosol. Collectively, our results suggest that plasma membrane cholesterol is important for both the intoxication mechanism of VacA and subsequent vacuole biogenesis.
Helicobacter pylori infection of the human stomach is a significant risk factor for the development of peptic ulcer disease and gastric cancer (34). H. pylori secretes a vacuolating cytotoxin (VacA) that induces degenerative vacuolation of mammalian cells both in vitro and in vivo (6, 24). VacA has been directly implicated in the epithelial erosion preceding the formation of both gastric and duodenal ulcers (13). Orally administered VacA causes gastric mucosal degeneration and inflammatory cell recruitment in mice, two key events in the development of gastric ulcer disease (54). Furthermore, only toxin-producing strains induce a pathology similar to that found in ulcer patients (25). Recently, VacA+ H. pylori strains were shown to outcompete VacA− strains in a mouse colonization model, suggesting that VacA may also be important for the establishment of H. pylori infection within a host (46). Despite considerable evidence that VacA is an important virulence factor in H. pylori pathogenesis, the mechanism of VacA-mediated cellular intoxication has not been determined.
Considerable evidence supports the hypothesis that VacA functions from an intracellular site of action (2, 57). The toxin binds and enters mammalian cells by a slow, temperature-dependent process (17). Indirect immunofluorescence studies have localized internalized VacA to both the cytosol (17) and membrane-bound vesicles (51). VacA also induces vacuole biogenesis when it is expressed directly within the cytosol of transiently transfected mammalian cells, further supporting the idea that the toxin acts intracellularly (9, 11, 62). Yeast two-hybrid analysis revealed interactions between VacA and a protein of unknown function that colocalizes with the intermediate filament protein vimentin (8). Recently, VacA was reported to translocate to the mitochondria and induce the release of cytochrome c (16). While these data collectively support an intracellular site of action for VacA, the mechanism of toxin entry into cells has not been elucidated.
The first step for VacA entry into target cells is the interaction of the toxin with the plasma membrane. Mature VacA is organized into amino- and carboxyl-terminal domains (p37 and p58, respectively) (7, 41, 48, 54), both of which are essential for toxin activity (9, 27, 54, 61, 62). Residues 480 to 700 of the toxin have been identified as residues that are especially important for cell line-specific recognition and binding (39, 55). Alternative methods employed to measure VacA binding to target cells have yielded contrasting results. When fluorescence-activated cell sorting was used, VacA binding to sensitive cell lines was reported to be saturable, suggesting the presence of a specific toxin receptor (26). However, at least three separate proteins have been implicated as the VacA receptor (37, 49, 58, 59). In contrast to fluorescence-activated cell sorting-based approaches, classical ligand-binding experiments performed with radiolabeled VacA indicated that the toxin may not bind specifically to a single cell surface component (28, 43). Several previous studies have shown that VacA readily associates with artificial lipid membranes in the absence of protein receptors (30, 31, 38). Collectively, these data imply that interactions between VacA and target cells are complex.
Identification of cellular determinants that are essential for vacuolation will likely yield critical information regarding the mechanism of VacA intoxication. Here, we investigated the importance of cholesterol for VacA-mediated cellular vacuolation. Cholesterol not only is a principal structural component of membranes but has also emerged as an important regulator of membrane trafficking within cells (21). Moreover, the importance of cholesterol for the cellular entry of toxins, as well as bacterial, viral, and parasitic pathogens, has been recently documented (1, 18, 50). Our results support the hypothesis that plasma membrane cholesterol is essential for cellular vacuolation induced by VacA.
MATERIALS AND METHODS
Materials.
Cell culture media, fetal bovine calf serum (FBS), neutral red solution, phosphate-buffered saline (PBS), penicillin-streptomycin, cell dissociation buffer, proteinase K solution (RNA grade), and trypsin-EDTA were purchased from Life Technologies, Inc. (Rockville, Md.). H. pylori strain 60190 (= ATCC 49503) and the HeLa (= ATCC CCL-2), CHO-K1 (= ATCC CCL-61), AGS (= ATCC CRL-1739), and Vero (= ATCC CCL-81) cell lines were received from the American Type Culture Collection (Manassas, Va.). Bisulfite-free brucella broth, vancomycin, methyl-β-cyclodextrin (MβCD), hydroxypropyl-β-cyclodextrin (HPCD), cholesterol (β-cyclodextrin [β-CD]-complexed soluble form), filipin, nystatin, saponin, digitonin, lovastatin, compactin, chlorpromazine, diphtheria toxin, cholera toxin, transferrin, an Infinity cholesterol reagent kit, bovine serum albumin (BSA), ovalbumin, polyvinylpyrrolidone (PVP), Triton X-100, anti-mouse immunoglobulin G-alkaline phosphatase conjugate, bromophenol blue, dithiothreitol, and most common laboratory chemicals and reagents were obtained from Sigma (St. Louis, Mo.); 25- and 75-cm2 plastic flasks, eight-well chamber slides, and 96-well plates were obtained from Corning (Cambridge, Mass.). The TNT T7 coupled reticulocyte lysate system, RNase inhibitor, 5-bromo-4-chloro-3-indolylphosphate (BCIP), and nitroblue tetrazolium were purchased from Promega (Madison, Wis.). [35S]methionine (1,175 Ci/mmol) and [3H]cholesterol (82 Ci/mmol) were obtained from NEN (Boston, Mass.). Scintiverse SX18-8 scintillation fluid was purchased from Fisher Scientific (Pittsburgh, Pa.). A LIVE/DEAD viability kit was acquired from Molecular Probes (Eugene, Oreg.). Mammalian protein extraction reagent (M-PER), Coomassie Plus protein assay reagent, protein G-Sepharose, IODO-GEN-precoated iodination tubes, a Micro Bio-Spin chromatography column, and the bicinchoninic acid (BCA) protein assay were purchased from Pierce (Rockford, Ill.). A CalPhos mammalian transfection kit was bought from Clontech Labs Inc. (Palo Alto, Calif.). 125Iodide (sodium salt; 16 Ci/mg) was purchased from Amersham Life Science (Arlington Heights, Ill.). Protease inhibitor cocktail set III was bought from Calbiochem (La Jolla, Calif.). Thin-layer chromatography (TLC) plates (Poly-gram Sil-G 60) were purchased from Whatman Inc. (Clifton, N.J.). Centricon 100 centrifugal microconcentrators were bought from Millipore (Bedford, Mass.).
Culturing H. pylori and purification of VacA.
H. pylori 700392 was cultivated as previously described (5). H. pylori was grown in culture flasks on a rotary platform shaker for 48 h to the stationary phase at 37°C under a 5% oxygen-5% carbon dioxide-90% nitrogen atmosphere in bisulfite- and sulfite-free brucella broth supplemented with 5% FBS and 5 μg of vancomycin/ml. H. pylori cultures were harvested by centrifugation at a relative centrifugal force of 7,500 by using a Sorvall RC2-B centrifuge at 4°C. VacA was initially fractionated and concentrated by ammonium sulfate (50%) precipitation. The ammonium sulfate-saturated pellet was resuspended and dialyzed into PBS (pH 7.2). Concentrated VacA was further purified by fast protein liquid chromatography by using a Superose 6 preparative grade resin in a HR16/50 column (Pharmacia, Piscataway, N.J.) previously equilibrated at 4°C in PBS (pH 7.2). Fast protein liquid chromatography fractions were analyzed for VacA by immunoblot analysis by using rabbit anti-VacA polyclonal antibodies (59). Fractions demonstrating cross-reacting material were further analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by Coomassie brilliant blue staining. The fractions demonstrating the greatest degree of purity were pooled and concentrated by using Centricon 100 centrifugal microconcentrators and stored at 4°C until use.
Activation of VacA.
VacA was acid activated as previously described by dropwise addition of 0.1 N HCl until the pH of the solution was approximately 3 (12). The solution was incubated for 30 min at 37°C and then reneutralized by dropwise addition of 300 mM NaOH until the solution pH was approximately 7.
Cell culture.
All mammalian cells were cultured as monolayers in 25-cm2 plastic flasks at 37°C under 5% CO2. HeLa cells were grown in 90% Dulbecco's minimal essential medium (DMEM). Vero cells were cultured in 90% Eagle's minimal essential medium with 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 1.5 g of sodium bicarbonate/liter. The CHO-K1 and AGS cell lines were cultured in 90% Ham's F-12 medium with 2 mM l-glutamine adjusted to contain 1.5 g of sodium bicarbonate/liter. All media were supplemented with 10% FBS, 100 U of penicillin/ml, and 100 mg of streptomycin/ml. Twenty-four hours prior to each experiment, 100 μl of cells was seeded into each well of a 96-well plate at a density of 1 × 105 cells/ml.
Manipulation of cell lines.
Mammalian cell lines were pretreated with different concentrations of each pharmacological agent for 1 h, unless otherwise noted. The following stock solutions were prepared: 400 mg of MβCD per ml in distilled water; 0.5 mg of HPCD per ml in distilled water; 50 mM β-CD-complexed cholesterol (water soluble) in distilled water; 5 mg of filipin per ml in methanol; 1 mg of saponin per ml in PBS; 1 mM digitonin in ethanol; 5 mg of nystatin per ml in dimethyl sulfoxide; 1 mM lovastatin in 50% ethanol; 10 mM compactin in ethanol; and 1 mg of chlorpromazine per ml in distilled water. Acid-activated VacA diluted in DMEM containing 5 mM NH4Cl was added to the cells and incubated at 37°C for 24 h. In some experiments, cells were preincubated with acid-activated VacA for 4 h at either 4 or 37°C and then for an additional 24 h with MβCD, HPCD, or β-CD-complexed cholesterol.
Analysis of cell vacuolation.
VacA-induced vacuolation of HeLa cells was assessed visually with an Olympus CK2 phase-contrast inverted microscope. Vacuolation was quantified based on the uptake of neutral red by mammalian cells as described previously (4). At the conclusion of each experiment, the medium was aspirated from the cell monolayers. The monolayers were incubated for 4 min with 100 μl of a neutral red solution (diluted 1:6 in DMEM) per well. After the cells were washed once with 200 μl of PBS/well, they were incubated with 100 μl of acidified ethanol (0.37% [vol/vol] HCl in 70% [vol/vol] ethanol). Neutral red uptake was determined by using a Dynatech MR5000 microtiter plate reader to measure the absorbance at 530 nm minus the absorbance at 410 nm.
The neutral red uptake readings for each well were normalized for total protein. Cell monolayers were washed once with 200 μl of PBS/well and lysed with 50 μl of M-PER/well. The total protein concentration was determined with the Coomassie Plus protein assay reagent by using BSA as a standard. The value for each well was normalized by dividing the neutral red uptake data by the total protein concentration.
Cholesterol quantification.
Cell monolayers were washed with PBS, detached with dissociation buffer, lysed in a buffer containing 0.1% SDS, 1 mM Na2EDTA, and 0.1 M Tris-HCl (pH 7.4), and homogenized by using a 19-gauge needle attached to a 1-ml syringe (19). The cholesterol content was determined by using the Infinity cholesterol reagent kit according to the instructions of the manufacturer. In each well, the total cholesterol content was normalized for total protein concentration by using the Coomassie Plus protein assay reagent.
Analysis of mammalian cell viability.
The combined LIVE/DEAD assay reagents were added to monolayers of HeLa cells grown in eight-well chamber slides according to the manufacturer's specifications. The cells were incubated for 30 to 45 min at room temperature, washed three times with PBS, and analyzed by visual inspection by using a fluorescence microscope (Olympus BX60) to count the numbers of dead and living cells.
In vitro transcription and translation of VacA.
35S-VacA was generated with the TNT T7 coupled reticulocyte lysate system used according to the manufacturer's specifications; pET20b-VacA was used as the template in the presence of [35S]methionine (specific activity, 1,175 Ci/mmol). pET20b-VacA harbors the gene encoding full-length, mature VacA (residues 1 to 821) downstream of the T7 promoter and replacing the NdeI-BamHI fragment of the parent plasmid, pET20b, as previously described (63). Each reaction mixture was analyzed by SDS-PAGE followed by imaging with a Fuji phosphorimager to determine the efficiencies of the coupled transcription-translation reactions. In vitro translated radiolabeled VacA was dialyzed three times at 4°C in binding buffer (50 mM HEPES [pH 7.2], 100 mM sodium chloride, 1 mM calcium chloride, 1 mM magnesium chloride, 100 mg of BSA/ml) and stored at −20°C. The VacA concentration was calculated by using the specific activity of radiolabeled methionine. Known concentrations of radiolabeled VacA and nonradiolabeled, purified VacA were acid activated and neutralized together (12).
Cell internalization assays.
Ninety-six-well plates were seeded with 100 μl of a suspension containing 1 × 105 HeLa cells/ml and incubated at 37°C for 24 h. HeLa cell monolayers were pretreated with MβCD or PBS (pH 7.2) for 1 h at 37°C. Acid-activated, radiolabeled VacA (50 nM), which was able to induce cellular vacuolation, and an equal volume of complete DMEM with 5 mM NH4Cl containing MβCD or PBS were added to HeLa cell monolayers. After incubation for 5 h at 37°C, the cell monolayers were washed three times with ice-cold 0.9% NaCl and treated with a freshly prepared 250-μg/ml proteinase K solution for 30 min at 4°C. Proteolyzed cells were immediately placed in boiling water for 5 min to inactivate the proteinase K. Radioactivity was quantified by scintillation counting with a LS-6001C Beckman model 15617C scintillation counter. The total protein in each well was quantified with the Coomassie Plus protein assay reagent. Samples were analyzed by SDS-PAGE and subsequent visualization with a Fuji phosphorimager.
Cell binding assays.
Ninety-six-well plates were seeded with 100 μl of a suspension containing 1 × 105 HeLa cells/ml and incubated at 37°C for 24 h. HeLa cell monolayers were pretreated with either MβCD or PBS (pH 7.2) for 1 h at 37°C before incubation at 4°C for 1 h. Chilled, acid-activated, radiolabeled VacA and an equal volume of prechilled complete DMEM with 5 mM NH4Cl containing MβCD or PBS were added to the monolayers. After incubation for 4 h at 4°C, the cells were washed rapidly three times with ice-cold binding buffer and lysed in 100 μl of M-PER. Radioactivity was quantified by scintillation counting of the samples resuspended in 2 ml of scintillation fluid. The recovered radiation was normalized for the total protein concentration in each well.
Radioiodination of VacA.
Purified VacA was radiolabeled with 125I by using IODO-GEN precoated iodination tubes as specified by the manufacturer. Radiolabeled VacA was separated from free iodine by using a Micro Bio-Spin chromatography column. Protein concentrations were determined by the micro-BCA assay, and the specific activity of labeling was quantified by using an Apex automatic gamma counter (model 28023; American Biomedical Consultants, Smithville, Mo.).
Detergent extraction of cultured cells.
HeLa cell monolayers (1 × 105 cells/ml) were prechilled to 4°C and incubated with prechilled 125I-VacA (0.05 to 25 μM) at 4°C. After 4 h, HeLa cells were detached by using dissociation buffer and collected by centrifugation at 300 × g for 5 min. Cells were resuspended in 0.5 ml of cold TNE buffer (25 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA) supplemented with 1% Triton X-100 and 25 μl of protease inhibitor cocktail set III per ml. The cells were incubated for 24 h at 4°C and centrifuged for 10 min at 16,000 × g at 4°C. The radioactivity in the supernatant (detergent-soluble fraction) and the radioactivity in the pellet (detergent-insoluble fraction) were quantified by gamma counting.
Sucrose density centrifugation.
Confluent monolayers of HeLa cells were incubated with 125I-VacA (1 to 25 μM) in complete DMEM (pH 7.4) for 4 h at 4°C. The cells were washed three times with ice-cold PBS, detached by using dissociation buffer, and collected by centrifugation at 300 × g for 5 min. The cells were resuspended in 0.5 ml of ice-cold TNE buffer supplemented with 1% Triton X-100 and 25 μl of protease inhibitor cocktail set III per ml. The membranes were solubilized by rotary shaking at 4°C overnight. Detergent-resistant membranes (DRMs) were purified with a step sucrose gradient. The sucrose concentration in the cell lysate was adjusted to 41% (in 10 mM Tris-HCl, pH 7.4), and the lysate was loaded into the bottom of an SW40 Beckman tube and overlaid with 8 ml of 35% sucrose, which was subsequently overlaid with 15% sucrose. After centrifugation at 35,000 rpm using a Beckman XL-80 ultracentrifuge with an SW40 rotor for 18 h at 4°C, 12 1-ml fractions were collected from the top to the bottom of the sucrose gradient. The radioactivity in each fraction was quantified by gamma counting.
VacA coimmunoprecipitation with cholesterol.
VacA-cholesterol coimmunoprecipitations were performed by using a previously described method (20). VacA (0.1 to 5 μM) or buffer alone was incubated with [3H]cholesterol (25 to 100 nM) for 2 h at room temperature with agitation. The samples were centrifuged at 18,000 × g for 5 min at 4°C. The supernatants were incubated with anti-VacA antibodies (59) for 1 h at 4°C with agitation. Protein G-Sepharose (15 μl) was then added, and incubation was continued for an additional 1 h at 4°C, again with agitation. The samples were centrifuged at 18,000 × g for 10 min at 4°C, and the supernatants were discarded. The pellets were washed four times with 1 ml of PBS and then resuspended in 100 μl of SDS-PAGE sample buffer (0.28 M Tris-HCl [pH 6.8], 30% glycerol, 1% SDS, 0.5 M dithiothreitol, 0.012% bromophenol blue). The samples were heated for 10 min at 95°C, and the beads were pelleted by centrifugation at 18,000 × g for 5 min. The radioactivity contained in 90 μl of the supernatant was determined by liquid scintillation counting. The remaining 10 μl was analyzed by SDS-PAGE and immunoblotting by using VacA antiserum (17) to verify that VacA was precipitated. Control experiments were performed in the absence of VacA. Each immunoprecipitation was performed in triplicate, and the entire experiment was repeated four times.
TLC overlay assay.
We used a TLC overlay assay adapted from a previously described method (53). Different concentrations of pure cholesterol (0.1 to 5 mg/ml) were spotted onto TLC plates. The plates were dried and blocked with 1% ovalbumin in 1% PVP-PBS. After incubation for 3 h at room temperature, the plates were incubated with either activated or unactivated 20 μM VacA or with PBS alone. After overnight incubation at 4°C, the plates were washed twice with PBS and incubated with a 1:2,000 dilution of anti-VacA antibodies in 3% PVP-PBS for 24 h at 37°C. The plates were washed twice with PBS and then blocked with 1% ovalbumin for 30 min at room temperature. The plates were washed again and incubated with a 1:10,000 dilution of anti-mouse immunoglobulin G-alkaline phosphatase conjugate for 1 h at 37°C. The bound conjugate was detected colorimetrically by incubating the plates in 100 mM Tris-HCl (pH 8.8)-150 mM NaCl-1 mM MgCl2 containing nitroblue tetrazolium-BCIP at 37°C. The enzymatic reaction was stopped by lowering the pH of the solution. This experiment was repeated five times.
Cholesterol antagonism of VacA association with HeLa cells.
Acid-activated 125I-VacA (0.1 to 4 μM) was mixed with pure cholesterol (100 μg/ml) or PBS and incubated at 37°C for 1 h with agitation. The cholesterol-VacA mixture was chilled to 4°C and added to prechilled HeLa cells. After incubation for 4 h at 4°C, the cells were washed rapidly three times with ice-cold wash buffer and lysed in 100 μl of M-PER. Radioactivity was quantified by gamma counting. The total protein concentration in each well was quantified by the BCA method. Each binding reaction was performed in quadruplicate, and the entire experiment was repeated at least four times.
Transfection of HeLa cells.
HeLa cells were transfected with pET20b plasmids expressing VacA under control of the T7 promoter (63). HeLa cells were first infected with recombinant vaccinia virus (vT7) bearing the T7 RNA polymerase gene (15). Vaccinia virus stock was trypsinized at 37°C for 30 min with vigorous vortexing every 5 to 10 min, and then 100 μl was added to HeLa cells (11). After infection for 30 min at 37°C, the virus stock was removed, and HeLa cells were transfected with plasmid DNA precipitates diluted in DMEM containing 2.5% FBS at 37°C. The plasmid DNA precipitates were prepared by slowly adding 4 μg of plasmid DNA in 100 μl of 0.25 M CaCl2 to 100 μl of 2× HEPES-buffered saline with frequently vortexing and then incubating the preparation at room temperature for 25 min before use. Each DNA precipitate was diluted 1:10 in DMEM with 2.5% FBS, and 100 μl was added to each well. The cells were incubated for 2 to 4 h at 37°C, and the transfection reagents were removed. The cells were washed twice with 100 μl of PBS (pH 7.2) and then incubated at 37°C for 20 h in DMEM containing 2.5% FBS and 5 mM NH4Cl.
Statistical analysis.
Data analyses were conducted by using a Student paired t test. A P value of less that 0.05 was considered statistically significant.
RESULTS
Depletion of cellular membrane cholesterol blocks VacA-mediated vacuolation.
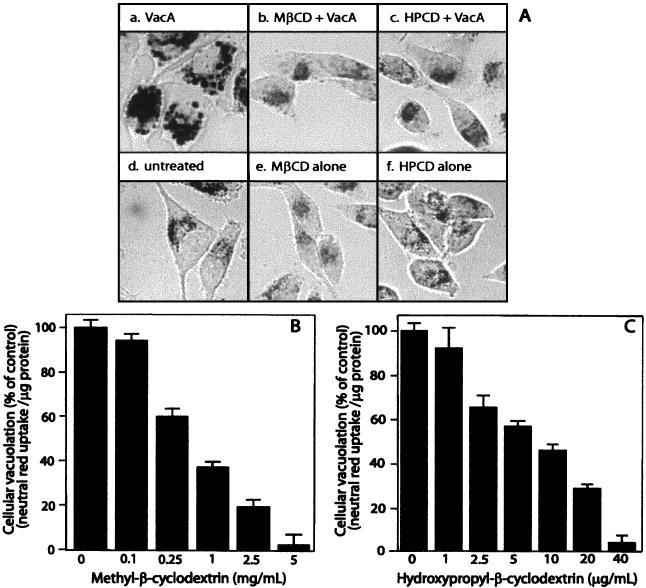
Monolayers of HeLa cells were incubated with purified VacA at 37°C. As previously reported (3, 24, 54), VacA induced the formation of small perinuclear vacuoles that were clearly evident after just 2 h. At the end of 24 h, the vacuoles were fully developed and filled the cytosol of nearly all the cells in the monolayers (Fig. 1A, panel a). To investigate the importance of plasma membrane cholesterol in VacA-mediated vacuolation, HeLa cell monolayers were pretreated for 1 h at 37°C with either 4 mg of MβCD/ml or 30 μg of HPCD/ml prior to toxin application. Both of these β-CDs selectively extract cholesterol from the plasma membrane without damaging cellular viability (36). In stark contrast to results obtained with VacA alone, MβCD or HPCD pretreatment completely blocked VacA-mediated cellular vacuolation, even after exposure of the monolayers to the toxin for 24 h (Fig. 1A, panels b and c).
FIG. 1.
MβCD and HPCD block HeLa cell sensitivity to VacA in a dose-dependent manner. HeLa cells were preincubated with either 4 mg of MβCD/ml or 30 μg of HPCD/ml for 1 h at 37°C and then incubated with acid-activated VacA (75 μg/ml). After 24 h, the HeLa cell monolayers were analyzed for vacuolation by phase-contrast microscopy (A) or quantification of neutral red uptake (B). (A) HeLa cells treated with VacA alone (panel a), MβCD plus VacA (panel b), HPCD plus VacA (panel c), PBS (panel d), MβCD (panel e), or HPCD (photograph f). (B and C) HeLa cell monolayers analyzed for vacuolation by determining uptake of neutral red, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
We confirmed that pretreatment of HeLa cells with either MβCD or HPCD for 1 h at 37°C lowered the overall cholesterol levels in the monolayers (Table 1). Neither MβCD nor HPCD was detectably cytotoxic to HeLa cell monolayers at concentrations that completely blocked VacA-mediated vacuolation (Fig. 1A, panels d, e, and f). To further confirm this observation, cells were treated for 1 h at 37°C with 4 mg of MβCD/ml or 30 μg of HPCD/ml and then tested for viability based on the uptake of calcein-AM (green fluorescence) or for loss of viability based on uptake of ethidium homodimer-1 (red fluorescence). Neither MβCD nor HPCD appreciably altered the relative number of viable cells in monolayers of HeLa cells (data not shown).
TABLE 1.
Cholesterol contents of HeLa cell monolayers
| Treatment | Cholesterol content (μg/mg)a | P value |
|---|---|---|
| Untreated cells | 29 ± 7.9 (100)b | |
| Cholesterol (100 μM) | 43 ± 10.4 (150) | 0.04 |
| MβCD (4 mg/ml) | 15 ± 3.1 (53) | 0.01 |
| HPCD (30 μg/ml) | 16 ± 5.1 (56) | 0.02 |
The cholesterol content is expressed as micrograms of cholesterol per milligram of total cell protein after 1 h of incubation with cholesterol, MβCD, or HPCD. The cholesterol content was calculated from the average of the results obtained in four separate experiments, each performed in triplicate. P values were determined for the cholesterol-, MβCD-, and HPCD-treated cells compared to the untreated cells.
The values in parentheses are percentages.
VacA-mediated vacuolation can be quantified by measuring the uptake of the acidotropic dye neutral red by intoxicated mammalian cells (6). HeLa cells that were pretreated with either MβCD or HPCD and then incubated for an additional 24 h with acid-activated VacA were assayed for neutral red uptake. HeLa cells pretreated with MβCD (Fig. 1B) or HPCD (Fig. 1C) prior to VacA application took up less neutral red than cells treated with VacA alone, indicating that vacuolation was inhibited. VacA-mediated cellular vacuolation, as quantified by neutral red uptake, was inhibited in a dose-dependent manner. Neutral red uptake was completely blocked when either 4 mg of MβCD/ml or 30 μg of HPCD/ml was used to pretreat HeLa cells.
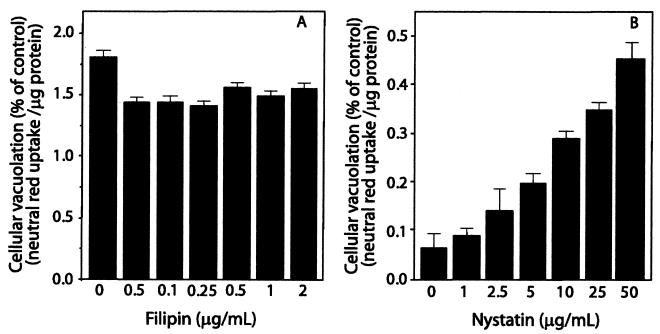
Depleting membrane cholesterol with β-CDs has been shown to reduce the number of caveolae and caveola-like structures at the plasma membrane (44), which require cholesterol to maintain their invaginated structural integrity (45). Sterol-binding reagents that do not extract cholesterol from the plasma membrane have also been shown to disrupt caveolae and caveola-dependent processes in HeLa cells (35). Because pretreatment of cells with β-CDs was shown to completely block cellular vacuolation, we next tested whether formation of cholesterol complexes with sterol-binding agents also inhibited the cellular action of VacA. HeLa cells were pretreated for 1 h with filipin (0.01 to 2 μg/ml), digitonin (0.1 to 5 μM), saponin (0.01 to 1 μg/ml), or nystatin (1 to 100 μg/ml) and then incubated for an additional 24 h after application of acid-activated VacA. In contrast to pretreatment of cells with MβCD or HPCD, pretreatment of HeLa cells with filipin (Fig. 2A), saponin, or digitonin (data not shown) had no apparent effect on VacA-mediated vacuolation at any of the concentrations tested. Moreover, preincubation for 1 h with nystatin strongly potentiated the extent of cellular vacuolation caused by VacA (Fig. 2B). Each of these findings indicates that the formation of cholesterol complexes at the plasma membrane with sterol-binding agents is not sufficient to block VacA-mediated cellular vacuolation.
FIG. 2.
Effects of cholesterol-binding agents on cellular vacuolation caused by VacA. HeLa cells were pretreated with different concentrations of filipin (A) or nystatin (B) for 1 h at 37°C and then incubated with acid-activated VacA (75 μg/ml). After 24 h, the HeLa cell monolayers were analyzed for vacuolation by determining uptake of neutral red, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
Because β-CDs extract cholesterol selectively from the plasma membrane, we next determined whether inhibiting the de novo biosynthesis of cholesterol at the endoplasmic reticulum also blocked vacuolation induced by VacA. HeLa cells were grown for 3 days in the presence of lovastatin (0.01 to 10 μM) and/or compactin (1 to 40 μM). The monolayers were incubated for an additional 24 h with acid-activated VacA and then assayed for neutral red uptake. In contrast to the effects of the β-CDs, pretreatment of cells with lovastatin or compactin had no detectable effect on VacA-mediated vacuolation (data not shown).
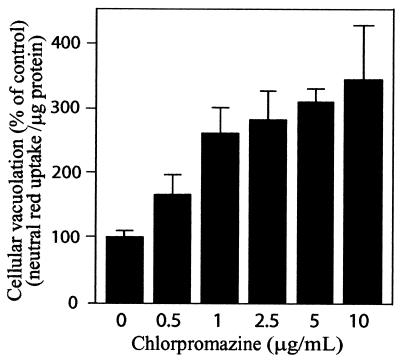
Extraction of membrane cholesterol with MβCD has recently been reported to disrupt clathrin-dependent endocytosis within HeLa cells, as well other mammalian cell lines (44, 52). To investigate whether β-CD blockage of VacA-mediated vacuolation was due to disruption of clathrin-mediated endocytosis, we tested whether the reagent chlorpromazine, which inhibits clathrin-coated pit assembly (56), also blocks VacA-mediated vacuolation. HeLa cells were pretreated for 1 h at 37°C with chlorpromazine, and then acid-activated VacA was applied for an additional 24 h. Both microscopic analysis (data not shown) and neutral red uptake measurements revealed that chlorpromazine strongly potentiated VacA-mediated vacuolation in a dose-dependent manner (Fig. 3). In control experiments, pretreatment of HeLa cells with chlorpromazine inhibited diphtheria toxin-mediated cytotoxicity (data not shown), which requires clathrin-mediated endocytosis for intoxication (33).
FIG. 3.
Chlorpromazine potentiates VacA-mediated vacuolation of HeLa cells. HeLa cells were pretreated with different concentrations of chlorpromazine for 1 h at 37°C and then incubated at 37°C with acid-activated VacA (25 μg/ml). After 24 h, the monolayers were analyzed for vacuolation by determining uptake of neutral red, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
Increasing the plasma membrane cholesterol level potentiates VacA-mediated vacuolation.
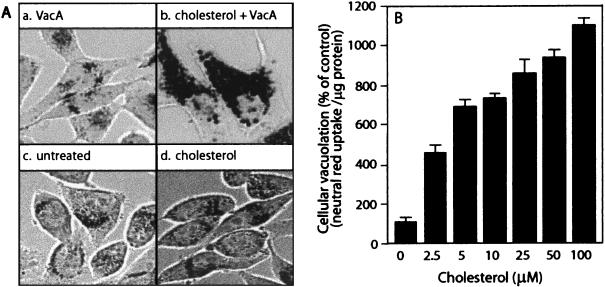
Because depleting plasma membrane cholesterol by β-CD pretreatment blocked vacuolation of HeLa cells by VacA, we hypothesized that cholesterol levels in the membrane are important for modulating VacA-mediated vacuolation. To test this hypothesis, we investigated the effects of increasing the plasma membrane cholesterol level on HeLa cells subsequently intoxicated with VacA. Monolayers of HeLa cells were pretreated with β-CD-complexed cholesterol for 1 h at 37°C prior to application of acid-activated VacA (60). Pretreating HeLa cells with cholesterol complexed to β-CD resulted in an increase in cellular cholesterol levels (Table 1) without detectable cytotoxicity or morphological changes in the monolayers (Fig. 4A, panels c and d). However, cholesterol pretreatment clearly potentiated cellular vacuolation caused by VacA. At low VacA concentrations that induced vacuole biogenesis in only 1 to 5% of the cells in a monolayer, pretreatment with β-CD-complexed cholesterol resulted in vacuolation of nearly the entire monolayer (Fig. 4A, panels a and b). Consistent with these observations, HeLa cells pretreated with β-CD-complexed cholesterol prior to VacA application exhibited a dose-dependent increase in neutral red uptake relative to the uptake in cells treated with VacA alone (Fig. 4B). Collectively, these results suggest that the plasma membrane cholesterol concentration modulates cellular vacuolation induced by VacA, with lower levels decreasing vacuolation and higher levels increasing vacuolation.
FIG. 4.
Cholesterol potentiates VacA-mediated vacuolation in a dose-dependent manner. HeLa cells were preincubated with 100 μM cholesterol for 1 h and then incubated with acid-activated VacA (25 μg/ml). After 24 h, the HeLa cell monolayers were analyzed for vacuolation by phase-contrast microscopy (A) or by quantification of neutral red uptake (B). (A) HeLa cells treated with VacA (panel a), cholesterol plus VacA (panel b), PBS (panel c), or cholesterol alone (panel d). (B) HeLa cells analyzed for neutral red uptake, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
Plasma membrane cholesterol modulates the vacuolation of multiple cell lines.
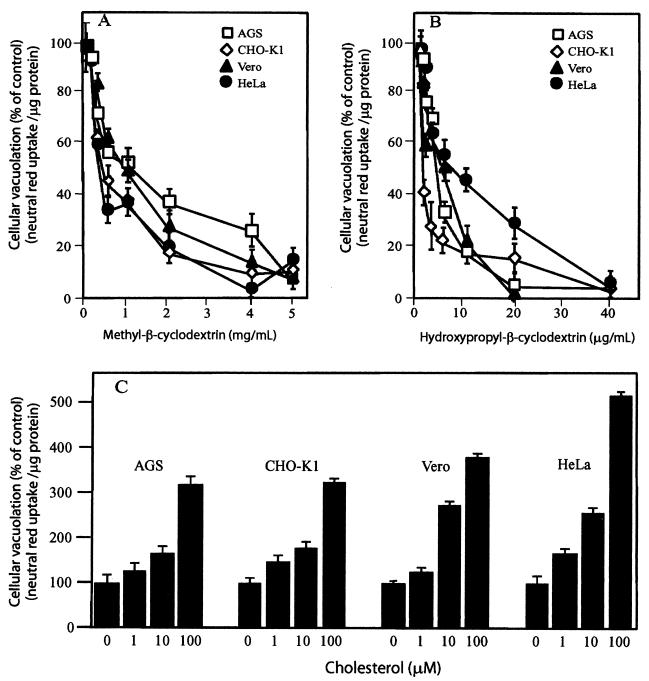
We next tested whether the importance of plasma membrane cholesterol levels to VacA-mediated cellular vacuolation observed in HeLa cells could be reproduced in other epithelium-derived cell lines. Notably, differences in cell line sensitivity for VacA have been reported previously (10, 39), presumably because of differences in the plasma membrane components required for cell binding and/or internalization. Monolayers of Vero, CHO-K1, HeLa, or stomach-derived AGS cells were pretreated with MβCD, HPCD, or β-CD-complexed cholesterol for 1 h at 37°C prior to application of acid-activated VacA. After 24 h, neutral red uptake was quantified, and the results revealed that VacA-mediated vacuolation in each cell line was blocked by MβCD (Fig. 5A) and HPCD (Fig. 5B) in a dose-dependent manner. These results were verified by phase-contrast microscopy, which revealed that there was complete blockage of vacuolation in each cell line pretreated with 4 mg of MβCD/ml or 30 μg of HPCD/ml (data not shown). Similarly, pretreatment of monolayers with β-CD-complexed cholesterol potentiated vacuolation in all the cell lines tested (Fig. 5C). These data indicate that plasma membrane cholesterol levels modulate vacuolation induced by VacA in multiple cell lines.
FIG. 5.
Cholesterol modulates the sensitivity of multiple cell lines to VacA. CHO-K1, Vero, HeLa, and AGS cells were preincubated with different concentrations of MβCD (A), HPCD (B), or cholesterol (C) for 1 h at 37°C and then incubated with acid-activated VacA. After 24 h, cells were analyzed for vacuolation by quantifying the uptake of neutral red, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
Plasma membrane cholesterol modulates VacA association with target cells.
To determine whether plasma membrane cholesterol directly influences the mechanism of VacA cellular intoxication, we investigated the effects of plasma membrane cholesterol depletion on the uptake of VacA into cells. HeLa cells were preincubated at 37°C with either 4 mg of MβCD/ml or PBS (pH 7.2) and then incubated at 37°C for 4 h with acid-activated 35S-radiolabeled VacA (50 nM), prepared as previously described (16). The cells were analyzed for VacA that was resistant to proteinase K treatment. As previously reported, VacA readily entered cells, as indicated by an SDS-PAGE analysis that revealed the presence of full-length VacA after proteinase K treatment (28, 43). To test whether depleting cholesterol from the plasma membrane affected internalization, the cells were pretreated with MβCD at a concentration of 4 mg/ml, a concentration that completely blocks VacA-mediated cellular vacuolation, and then for an additional 4 h with acid-activated VacA. Pretreatment of HeLa cell monolayers with MβCD was found to decrease recovery of radiolabeled VacA to less than 30% of the levels observed for control monolayers pretreated with VacA alone (data not shown), suggesting that depletion of membrane cholesterol reduced the entry of VacA into target cells.
It is possible that attenuation of VacA internalization may be due to a reduction in the association of VacA with MβCD-treated cells. Independent reports from two separate groups have indicated that VacA binds to mammalian cells in a nonspecific manner (28, 43). In agreement with these previous studies, we detected dose-dependent binding of radiolabeled VacA (0.5 to 50 nM) to HeLa cell monolayers (data not shown). Similar to the earlier reports, we also found that association of VacA (10 nM) with HeLa cells was not appreciably inhibited when the cells were coincubated with a 50-fold molar excess of nonradiolabeled VacA (data not shown). We extracted membrane cholesterol using concentrations of MβCD that completely blocked VacA-based vacuolation and tested for association of VacA with cells. Cells were pretreated with 4 mg of MβCD/ml for 1 h at 37°C and then chilled to 4°C for at least 1 h. Acid-activated radiolabeled VacA (50 nM) was allowed to bind for 4 h at 4°C. The cells were then washed extensively and lysed, and the recovered toxin was quantified by scintillation counting. These experiments indicated that pretreating cells with MβCD lowered the level of recovery of radiolabeled VacA to a level that was approximately 60% of the level observed for control monolayers pretreated with PBS alone (data not shown).
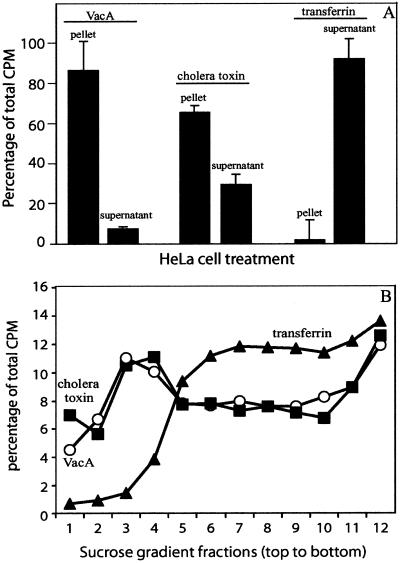
The demonstration that cholesterol-depleting drugs reduce VacA association with cultured mammalian cells suggests that VacA may directly associate with detergent-insoluble membrane microdomains that are enriched in cholesterol, called DRMs. To test this hypothesis, we incubated radiolabeled VacA with monolayers of HeLa cells prechilled to 4°C, as described above. After 4 h, the cells were extracted with 1.0% Triton X-100, and the radiation in the soluble fractions and the radiation in the insoluble fractions were separated by centrifugation and quantified. In control experiments, we analyzed HeLa cells pretreated with 125I-transferrin and 125I-cholera toxin, which have been shown to separate primarily to the detergent-soluble and -insoluble fractions, respectively. These experiments revealed that radiolabeled VacA separated predominantly to the insoluble fraction, like cholera toxin (Fig. 6A). In contrast, transferrin was detected predominantly in the soluble fraction.
FIG. 6.
Association of VacA with detergent-insoluble microdomains. HeLa cell monolayers were incubated with acid-activated 125I-radiolabeled VacA (50 nM) (○), cholera toxin (10 nM) (▪), or transferrin (10 μg/ml) (▴) in DMEM containing NH4Cl (5 mM). After incubation for 4 h at 4°C, the cells were washed three times with ice-cold PBS, detached, and collected by centrifugation at 300 × g for 5 min. The cells were resuspended in 0.5 ml of cold TNE buffer supplemented with 1% Triton X-100 and 25 μg of protease inhibitor cocktail set III per ml. Membranes were solubilized by rotary shaking at 4°C. (A) Radioactivity in the supernatant (detergent-soluble fraction) and in the pellet (detergent-insoluble fraction) quantified as described in Materials and Methods. (B) DRMs purified with a step sucrose gradient, as described in Materials and Methods. The values are the percentages of the total counts. The data are data from a representative experiment performed at least four times. The error bars indicate standard deviations.
To further investigate the idea that VacA associates with DRMs, we fractionated detergent-extracted cells by density gradient centrifugation. Radiolabeled VacA was incubated with monolayers of HeLa cells at 4°C for 4 h. The cells were detergent extracted with 1.0% Triton X-100, as described above, and were fractionated by using sucrose density gradients. Individual fractions were collected, and the radiation in each fraction was quantified. As demonstrated in Fig. 6B, cell-associated VacA was enriched in the fractions collected from the top of the sucrose density gradient. Control experiments revealed that cholera toxin, but not transferrin, was enriched at the top of the sucrose density gradient. Moreover, VacA alone (in the absence of cells) was not enriched in fractions collected from the top of the sucrose gradient (data not shown). Collectively, the demonstration that cell-associated VacA is detergent insoluble and floats on the top of sucrose density gradients suggests that VacA may directly associate with DRMs at the surfaces of mammalian cells.
Because VacA-mediated vacuolation is dependent on plasma membrane cholesterol levels and because cholesterol is a major component of DRMs, it is possible that VacA associates directly with cholesterol. We attempted to detect VacA-cholesterol interactions by using three distinct approaches, as described in Materials and Methods. First, we determined whether preincubating VacA with cholesterol would antagonize association of the toxin with target cells. Second, we tried to detect binding of VacA to purified cholesterol on TLC plates. Finally, we investigated whether radiolabeled cholesterol would coimmunoprecipitate with VacA. Repeated attempts in which all three approaches were used failed to demonstrate detectable interactions between VacA and cholesterol (data not shown). These results suggest that VacA may associate with DRM components other than (or in addition to) cholesterol.
Plasma membrane cholesterol modulates vacuole biogenesis.
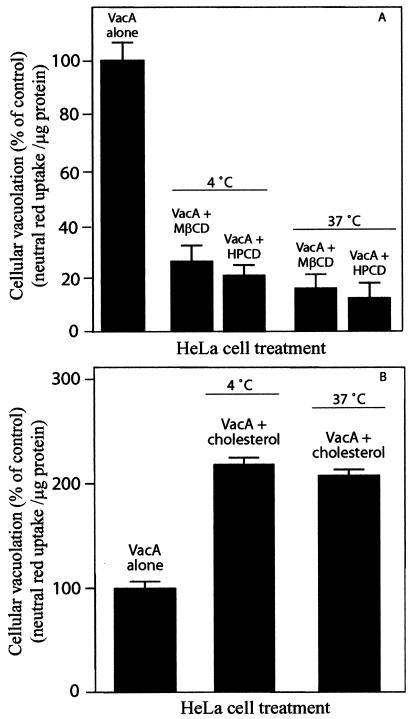
To further investigate the relationship of plasma membrane cholesterol to vacuolation, HeLa cells were preloaded with VacA and then treated with MβCD, HPCD, or β-CD-complexed cholesterol. In one experiment, acid-activated VacA was incubated for 4 h with prechilled HeLa cells (4°C). The cells were then extensively washed and incubated at 37°C for an additional 24 h in the presence of either 4 mg of MβCD/ml or 30 μg of HPCD/ml. Both visual inspection by phase-contrast microscopy (data not shown) and neutral red uptake measurements indicated that even after VacA was prebound to HeLa cells, MβCD and HPCD completely blocked vacuolation (Fig. 7A). In addition, cells treated with 100 μM β-CD-complexed cholesterol after VacA binding exhibited increased neutral red uptake relative to the uptake by cells incubated with VacA alone (Fig. 7B).
FIG. 7.
Effects of altering membrane cholesterol on vacuolation of cells pretreated with VacA. HeLa cells were preincubated with acid-activated (100 μg/ml) VacA for 4 h at either 4 or 37°C and then incubated for an additional 24 h with 4 mg of MβCD/ml or 30 μg of HPCD/ml (A). Alternatively, HeLa cells were preincubated with acid-activated (25 μg/ml) VacA for 4 h at either 4 or 37°C and then incubated for an additional 24 h with 100 μM cholesterol (B). The HeLa cell monolayers were analyzed for vacuolation by determining uptake of neutral red, as described in Materials and Methods. The data are data from a representative experiment performed at least in quadruplicate. The error bars indicate standard deviations.
Alternatively, HeLa cells were incubated with VacA for 4 h at 37°C prior to application of either 4 mg of MβCD/ml or 30 μg of HPCD/ml. The monolayers were incubated at 37°C for an additional 24 h and then analyzed for vacuolation. In these experiments MβCD and HPCD completely blocked vacuolation, as assessed by either phase-contrast microscopy (data not shown) or neutral red uptake (Fig. 7A). In addition, when cells were treated with 100 μM β-CD-complexed cholesterol after incubation at 37°C with VacA for 4 h, both microscopic examination and neutral red uptake data clearly demonstrated potentiation of vacuolation (Fig. 7B). These data suggest that modulating plasma membrane cholesterol also affects a step downstream of toxin association and internalization.
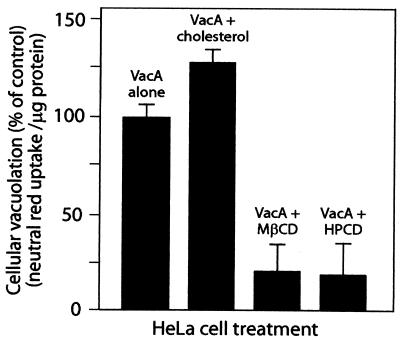
VacA has been shown to induce cellular vacuolation when it is expressed directly in mammalian cells by using a transient transfection system (9, 11, 62). We employed this approach, which bypasses the need for the toxin to be internalized from the outside of the cell, to further investigate the importance of plasma membrane cholesterol in vacuolation. HeLa cells that had been previously infected with vT7 vaccinia virus expressing T7 polymerase were transfected with pET20b-VacA harboring the gene encoding full-length VacA under control of the T7 polymerase promoter (14, 62). MβCD (4 mg/ml) or HPCD (30 μg/ml) was applied to cells 4 h after transfection, and the monolayer was incubated at 37°C. After 24 h, neutral red uptake was measured. HeLa cell monolayers transfected with plasmids expressing VacA exhibited vacuolation in 60 to 70% of the cells and a detectable increase in neutral red uptake. However, both MβCD and HPCD completely blocked cellular vacuolation, as visualized by phase-contrast microscopy (data not shown) and neutral red uptake (Fig. 8). In addition, when cells were treated with β-CD-complexed cholesterol (100 μM) after transfection, an increase in neutral red uptake was observed (Fig. 8). Significantly, these data suggest that in addition to affecting the cellular intoxication mechanism of the toxin, plasma membrane cholesterol is important for vacuole biogenesis.
FIG. 8.
MβCD and HPCD block vacuolation of transiently transfected cells expressing VacA. HeLa cells were transfected with pET20b plasmids expressing VacA, as described in Materials and Methods. After 4 h, 4 mg of MβCD/ml, 30 μg of HPCD/ml, or 100 μM cholesterol was applied to the monolayers, which were then incubated at 37°C under 5% CO2. After 20 h, the cells were assayed for uptake of neutral red. Data are expressed as percentages of neutral red uptake by treated cells relative to the neutral red uptake by HeLa cells transfected with a plasmid expressing full-length VacA-green fluorescent protein but not treated following transfection. The data from two separate experiments performed at least in quadruplicate were averaged. The error bars indicate standard deviations.
DISCUSSION
Cholesterol is essential for regulating the properties of mammalian cell membranes. Over the past few years, the importance of cholesterol homeostasis to lipid trafficking and sorting in cells has become evident (21). Investigations into the effects of modulating the levels of plasma membrane cholesterol with β-CDs have revealed the importance of this sterol to endocytic mechanisms involving vesicular trafficking (44, 52). Because VacA is believed to enter mammalian cells by an endocytic mechanism (17, 28, 43) and subsequently induces vacuole formation by presumably altering vesicle trafficking (29, 40), we wanted to test whether cholesterol-dependent endocytic events are linked to the VacA intoxication mechanism and/or vacuole biogenesis. The importance of cholesterol to pathogenesis has been underscored by recent findings that this sterol is critical for cellular entry of toxins (19, 47), as well as a number of pathogens, including bacteria (18), parasites (32), and viruses (1)
In this study, we demonstrated that plasma membrane cholesterol is essential for vacuolation of mammalian cells by VacA. Collectively, the finding that vacuolation could be blocked by lowering cholesterol levels and the finding that vacuolation could be potentiated by increasing cholesterol levels suggested that the cholesterol concentration within the membrane is a critical factor for the extent of vacuole biogenesis induced by VacA. The importance of cholesterol localized specifically to the plasma membrane is supported by the fact that the β-CDs exchange cholesterol selectively at the cell surface (42) and by our finding that blocking the de novo synthesis of cholesterol at the endoplasmic reticulum had no detectable effect on VacA-mediated vacuolation.
Depleting membrane cholesterol with β-CDs has been reported to disrupt cholesterol and sphingolipid-rich microdomains called lipid rafts or DRMs (23). DRMs can be localized within caveolae but have also been reported to be present in cells deficient in caveolae (22). Cholesterol is essential for the structure and function of invaginated caveolae, as well as caveola-dependent endocytosis (45). β-CDs directly disrupt caveola structures at the plasma membrane, presumably by facilitating the disassembly of lipid rafts within the caveolae (44). Because cholesterol depletion inhibits VacA-mediated vacuolation, we hypothesized that caveola-dependent endocytosis might be important for toxin internalization and/or targeting of vesicles required for vacuole biogenesis. However, cholesterol-binding reagents, such as filipin, which is known to disrupt cholesterol-rich microdomains localized to caveolae and caveola-like structures (45) and has been shown to block caveola-mediated entry of a number of bacterial pathogens into HeLa cells (35), did not exert a detectable effect on VacA-mediated cellular vacuolation. Thus, the inhibitory effects of the β-CDs cannot readily be interpreted to be a direct result of the disruption of caveolae, and the role, if any, of caveola-mediated vesicular transport in VacA cellular activity requires further investigation.
Depletion of membrane cholesterol with MβCD was recently demonstrated to strongly attenuate clathrin-dependent endocytosis (44, 52). Moreover, supplementing membrane cholesterol levels resulted in recovery of clathrin-mediated endocytosis that had previously been blocked with MβCD (44). Thus, we hypothesized that β-CDs may block cellular vacuolation by inhibiting clathrin-mediated endocytic processes involved in VacA internalization and/or vacuole biogenesis. However, pretreatment of HeLa cell monolayers with chlorpromazine, which disrupts endocytosis involving clathrin-coated vesicles (56), did not have an inhibitory effect on vacuole biogenesis. Our finding that VacA-induced vacuolation is not dependent on a clathrin-mediated endocytic mechanism is in agreement with earlier work showing that transfection of mammalian cells with dominant-negative proteins that exert an inhibitory effect on clathrin-dependent endocytosis did not alter the extent of vacuolation within monolayers intoxicated with VacA (43).
While our results support the hypothesis that vacuolation of mammalian cells by VacA does not require functional caveola- or clathrin-dependent endocytosis, it is noteworthy that cholesterol is also important for alternative endocytic processes, collectively termed clathrin-independent endocytosis (47). VacA induction of vacuolation requires that the toxin binds and enters cells by a slow, temperature-dependent process (17, 28, 43) and then functions from an intracellular site of action. Our results indicated that depletion of plasma membrane cholesterol with MβCD decreased the amount of VacA internalized into HeLa cells by more than 70% and also reduced association of the toxin with cultured cells. Our data indicate that cell-bound VacA is associated with DRMs, suggesting that VacA may directly associate with cholesterol-rich microdomains. It is not clear whether VacA directly associates with cholesterol or, alternatively, whether cholesterol is important for the ordering of plasma membrane components needed for productive toxin binding and cell entry. Attempts to demonstrate that VacA directly associates with cholesterol were unsuccessful, suggesting that VacA may bind to DRM components other than (or in addition to) cholesterol. Treatment of HEp-2 cells with phosphatidylinositol phospholipase C has been shown to inhibit VacA-mediated vacuolation and internalization of the toxin, suggesting that one or more glycosylphosphatidylinositol-anchored proteins within DRMs may be important for VacA intoxication (43). While our results support the hypothesis that VacA entry into cells requires plasma membrane cholesterol, the inability to block vacuolation by disrupting caveola- or clathrin-dependent entry into the cell suggests that VacA may enter cells by one or more clathrin-independent endocytic pathways.
Notably, our results also imply that plasma membrane cholesterol is important for vacuole biogenesis. Even after VacA is preloaded and incubated with cells under conditions that have been shown to promote cellular entry of the toxin, depleting the membrane cholesterol blocked vacuolation in a monolayer. Moreover, in transiently transfected cells in which VacA was expressed directly within the cytosol in order to bypass the membrane binding and internalization steps of intoxication, vacuolation was also blocked by cholesterol depletion. Collectively, these results indicate that depleting membrane cholesterol disrupts an essential process for vacuole biogenesis, subsequent to toxin entry. Whether this is clathrin-independent vesicular transport or an alternative process remains to be elucidated.
In summary, we have established that membrane cholesterol is an important host cell component that modulates the extent of cellular vacuolation caused by VacA. Our data suggest that plasma membrane cholesterol may be important for the cellular activity of VacA by regulating entry of toxin into sensitive cells and then modulating cellular processes that are essential for vacuole biogenesis. Identification of the exact role of cholesterol in the mechanism of VacA-mediated cellular vacuolation may provide novel insights into toxin trafficking pathways and mechanisms of vacuole biogenesis.
Acknowledgments
We thank P. Hardin for use of the fluorescence microscope and also A. Vailas and D. Martinez, who provided access to their laboratory's microtiter plate reader. P. Roch is acknowledged for assistance with preparation of the manuscript, and Jessica Feith is acknowledged for initial characterization of cellular cholesterol levels.
This work was supported by the National Institutes of Health (grant AI45928), the Robert A. Welch Foundation (grant E-1311), and the American Heart Association (grant BG472).
Editor: J. T. Barbieri
REFERENCES
- 1.Bernardes, C., A. Antonio, M. C. Pedroso de Lima, and M. L. Valdeira. 1998. Cholesterol affects African swine fever virus infection. Biochim. Biophys. Acta 1393:19-25. [DOI] [PubMed] [Google Scholar]
- 2.Cover, T. L. 1998. An intracellular target for Helicobacter pylori vacuolating toxin. Trends Microbiol. 6:127-128. [DOI] [PubMed] [Google Scholar]
- 3.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 4.Cover, T. L., S. A. Halter, and M. J. Blaser. 1992. Characterization of HeLa cell vacuoles induced by Helicobacter pylori broth culture supernatant. Hum. Pathol. 23:1004-1010. [DOI] [PubMed] [Google Scholar]
- 5.Cover, T. L., P. I. Hanson, and J. E. Heuser. 1997. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J. Cell Biol. 138:759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover, T. L., W. Puryear, G. I. Perez-Perez, and M. J. Blaser. 1991. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect. Immun. 59:1264-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover, T. L., M. K. Tummuru, P. Cao, S. A. Thompson, and M. J. Blaser. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 269:10566-10573. [PubMed] [Google Scholar]
- 8.de Bernard, M., M. Moschioni, G. Napolitani, R. Rappuoli, and C. Montecucco. 2000. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 19:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bernard, M., D. Burroni, E. Papini, R. Rappuoli, J. Telford, and C. Montecucco. 1998. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect. Immun. 66:6014-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bernard, M., M. Moschioni, E. Papini, J. Telford, R. Rappuoli, and C. Montecucco. 1998. Cell vacuolization induced by Helicobacter pylori VacA toxin: cell line sensitivity and quantitative estimation. Toxicol. Lett. 99:109-115. [DOI] [PubMed] [Google Scholar]
- 11.de Bernard, M., B. Arico, E. Papini, R. Rizzuto, G. Grandi, R. Rappuoli, and C. Montecucco. 1997. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol. Microbiol. 26:665-674. [DOI] [PubMed] [Google Scholar]
- 12.de Bernard, M., E. Papini, V. de Filippis, E. Gottardi, J. Telford, R. Manetti, A. Fontana, R. Rappuoli, and C. Montecucco. 1995. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J. Biol. Chem. 270:23937-23940. [DOI] [PubMed] [Google Scholar]
- 13.Figura, N., P. Guglielmetti, A. Rossolini, A. Barberi, G. Cusi, R. A. Musmanno, M. Russi, and S. Quaranta. 1989. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J. Clin. Microbiol. 27:225-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., P. L. Earl, and B. Moss. 1987. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol. Cell. Biol. 7:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner, J. A., and T. L. Cover. 1996. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64:4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 19.Grimmer, S., T. G. Iversen, B. van Deurs, and K. Sandvig. 2000. Endosome to Golgi transport of ricin is regulated by cholesterol. Mol. Biol. Cell 11:4205-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodel, A., S. J. An, N. J. Hansen, J. Lawrence, B. Wasle, M. Schrader, and J. M. Edwardson. 2001. Cholesterol-dependent interaction of syncollin with the membrane of the pancreatic zymogen granule. Biochem. J. 356:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoekstra, D., and I. S. C. van. 2000. Lipid trafficking and sorting: how cholesterol is filling gaps. Curr. Opin. Cell Biol. 12:496-502. [DOI] [PubMed] [Google Scholar]
- 22.Hooper, N. M. 1999. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol. Membr. Biol. 16:145-156. [DOI] [PubMed] [Google Scholar]
- 23.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leunk, R., P. Johnson, B. David, W. Kraft, and D. Morgan. 1988. Cytotoxin activity in broth culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 25.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 26.Massari, P., R. Manetti, D. Burroni, S. Nuti, N. Norais, R. Rappuoli, and J. L. Telford. 1998. Binding of the Helicobacter pylori vacuolating cytotoxin to target cells. Infect. Immun. 66:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClain, M., P. Cao, and T. Cover. 2001. Amino-terminal hydrophobic region of Helicobacter pylori vacuolating cytotoxin (VacA) mediates transmembrane protein dimerization. Infect. Immun. 69:1181-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain, M., W. Schraw, V. Ricci, P. Boquet, and T. Cover. 2000. Acid activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol. Microbiol. 37:422-442. [DOI] [PubMed] [Google Scholar]
- 29.Molinari, M., C. Galli, N. Norais, J. L. Telford, R. Rappuoli, J. P. Luzio, and C. Montecucco. 1997. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J. Biol. Chem. 272:25339-25344. [DOI] [PubMed] [Google Scholar]
- 30.Molinari, M., C. Galli, M. de Bernard, N. Norais, J. M. Ruysschaert, R. Rappuoli, and C. Montecucco. 1998. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem. Biophys. Res. Commun. 248:334-340. [DOI] [PubMed] [Google Scholar]
- 31.Moll, G., E. Papini, R. Colonna, D. Burroni, J. Telford, R. Rappuoli, and C. Montecucco. 1995. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur. J. Biochem. 234:947-952. [DOI] [PubMed] [Google Scholar]
- 32.Mordue, D. G., N. Desai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moya, M., A. Dautry-Varsat, B. Goud, D. Louvard, and P. Boquet. 1985. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J. Cell Biol. 101:548-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. 1994. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA 272:65-69. [PubMed] [Google Scholar]
- 35.Norkin, L. C., S. A. Wolfrom, and E. S. Stuart. 2001. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 266:229-238. [DOI] [PubMed] [Google Scholar]
- 36.Ohtani, Y., T. Irie, K. Uekama, K. Fukunaga, and J. Pitha. 1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186:17-22. [DOI] [PubMed] [Google Scholar]
- 37.Padilla, P. I., A. Wada, K. Yahiro, M. Kimura, T. Niidome, H. Aoyagi, A. Kumatori, M. Anami, T. Hayashi, J. Fujisawa, H. Saito, J. Moss, and T. Hirayama. 2000. Morphologic differentiation of HL-60 cells is associated with appearance of RPTP-beta and induction of Helicobacter pylori VacA sensitivity. J. Biol. Chem. 275:15200-15206. [DOI] [PubMed] [Google Scholar]
- 38.Pagliaccia, C., X. M. Wang, F. Tardy, J. L. Telford, J. M. Ruysschaert, and V. Cabiaux. 2000. Structure and interaction of VacA of Helicobacter pylori with a lipid membrane. Eur. J. Biochem. 267:104-109. [DOI] [PubMed] [Google Scholar]
- 39.Pagliaccia, C., M. de Bernard, P. Lupetti, X. Ji, D. Burroni, T. L. Cover, E. Papini, R. Rappuoli, J. L. Telford, and J. M. Reyrat. 1998. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc. Natl. Acad. Sci. USA 95:10212-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papini, E., M. de Bernard, E. Milia, M. Bugnoli, M. Zerial, R. Rappuoli, and C. Montecucco. 1994. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA 91:9720-9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phadnis, S. H., D. Ilver, L. Janzon, S. Normark, and T. U. Westblom. 1994. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect. Immun. 62:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitha, J., T. Irie, P. Sklar, and J. Nye. 1988. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 43:493-502. [DOI] [PubMed] [Google Scholar]
- 43.Ricci, V., A. Galmiche, A. Doye, V. Necchi, E. Solcia, and P. Boquet. 2000. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 11:3897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothberg, K. G., Y. S. Ying, B. A. Kamen, and R. G. Anderson. 1990. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol. 111:2931-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69:730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandvig, K., and B. van Deurs. 1999. Endocytosis and intracellular transport of ricin: recent discoveries. FEBS Lett. 452:67-70. [DOI] [PubMed] [Google Scholar]
- 48.Schmitt, W., and R. Haas. 1994. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 12:307-319. [DOI] [PubMed] [Google Scholar]
- 49.Seto, K., Y. Hayashi-Kuwabara, T. Yoneta, H. Suda, and H. Tamaki. 1998. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 431:347-350. [DOI] [PubMed] [Google Scholar]
- 50.Shin, J. S., and S. N. Abraham. 2001. Co-option of endocytic functions of cellular caveolae by pathogens. Immunology 102:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sommi, P., V. Ricci, R. Fiocca, V. Necchi, M. Romano, J. L. Telford, E. Solcia, and U. Ventura. 1998. Persistence of Helicobacter pylori VacA toxin and vacuolating potential in cultured gastric epithelial cells. Am. J. Physiol. 275:G681-G688. [DOI] [PubMed] [Google Scholar]
- 52.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H. Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, Y., T. Nakao, T. Ito, N. Watanabe, Y. Toda, G. Xu, T. Suzuki, T. Kobayashi, Y. Kimura, A. Yamada, K. Sugawara, H. Nishimura, F. Kitame, K. Nakamura, E. Deya, M. Kiso, and A. Hasagawa. 1992. Structural determination of gangliosides that bind to influenza a, b, and c viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology 189:121-131. [DOI] [PubMed] [Google Scholar]
- 54.Telford, J. L., P. Ghiara, M. Dell'Orco, M. Comanducci, D. Burroni, M. Bugnoli, M. F. Tecce, S. Censini, A. Covacci, Z. Xiang, E. Papini, C. Montecucco, L. Parente, and R. Rappuoli. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med. 179:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, H. J., and W. C. Wang. 2000. Expression and binding analysis of GST-VacA fusions reveals that the C-terminal approximately 100-residue segment of exotoxin is crucial for binding in HeLa cells. Biochem. Biophys. Res. Commun. 278:449-454. [DOI] [PubMed] [Google Scholar]
- 56.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willhite, D. C., A. Gutierrez, D. Ye, C. L. Williams, H. K. Patel, K. B. Marty, and S. R. Blanke. 2000. Intracellular life and times of the Helicobacter pylori vacuolating toxin. SAAS Bull. Biochem. Biotechnol. 13:35-53. [Google Scholar]
- 58.Yahiro, K., T. Niidome, T. Hatakeyama, H. Aoyagi, H. Kurazono, P. I. Padilla, A. Wada, and T. Hirayama. 1997. Helicobacter pylori vacuolating cytotoxin binds to the 140-kDa protein in human gastric cancer cell lines, AZ-521 and AGS. Biochem. Biophys. Res. Commun. 238:629-632. [DOI] [PubMed] [Google Scholar]
- 59.Yahiro, K., T. Niidome, M. Kimura, T. Hatakeyama, H. Aoyagi, H. Kurazono, K. Imagawa, A. Wada, J. Moss, and T. Hirayama. 1999. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J. Biol. Chem. 274:36693-36699. [DOI] [PubMed] [Google Scholar]
- 60.Yancey, P. G., W. V. Rodrigueza, E. P. Kilsdonk, G. W. Stoudt, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1996. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271:16026-16034. [DOI] [PubMed] [Google Scholar]
- 61.Ye, D., and S. R. Blanke. 2000. Mutational analysis of the Helicobacter pylori vacuolating toxin amino terminus: identification of amino acids essential for cellular vacuolation. Infect. Immun. 68:4354-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye, D., D. C. Willhite, and S. R. Blanke. 1999. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J. Biol. Chem. 274:9277-9282. [DOI] [PubMed] [Google Scholar]